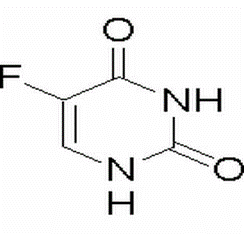
5-Fluorouracil(CAS# 51-21-8)
| Risk Codes | R22 – Harmful if swallowed R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R52 – Harmful to aquatic organisms R25 – Toxic if swallowed |
| Safety Description | S36 – Wear suitable protective clothing. S36/37 – Wear suitable protective clothing and gloves. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S22 – Do not breathe dust. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | YR0350000 |
| FLUKA BRAND F CODES | 10-23 |
| TSCA | T |
| HS Code | 29335995 |
| Hazard Note | Irritant/Highly Toxic |
| Hazard Class | 6.1 |
| Packing Group | III |
| Toxicity | LD50 orally in Rabbit: 230 mg/kg |
Introduction
This product is first converted into 5-fluoro-2-deoxyuracil nucleotides in the body, which inhibits thymine nucleotide synthase and blocks the conversion of deoxyuracil nucleotides into deoxythymine nucleotides, thereby inhibiting DNA biosynthesis. In addition, by preventing the incorporation of uracil and rotic acid into RNA, the effect of inhibiting RNA synthesis is achieved. This product is a cell cycle specific drug, mainly inhibiting S phase cells.
Write your message here and send it to us