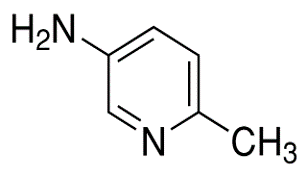
5-Amino-2-methylpyridine(CAS# 3430-14-6)
| Risk Codes | R22 – Harmful if swallowed R37/38 – Irritating to respiratory system and skin. R41 – Risk of serious damage to eyes R36/37/38 – Irritating to eyes, respiratory system and skin. R34 – Causes burns R24/25 - |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/39 - S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S36 – Wear suitable protective clothing. S27 – Take off immediately all contaminated clothing. S36/37 – Wear suitable protective clothing and gloves. |
| UN IDs | UN2811 |
| WGK Germany | 3 |
| HS Code | 29333999 |
| Hazard Note | Harmful |
| Hazard Class | 6.1 |
| Packing Group | III |
Introduction
6-Methyl-3-aminopyridine is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of 6-methyl-3-aminopyridine:
Quality:
Appearance: 6-methyl-3-aminopyridine is a colorless or yellowish crystal.
Solubility: It has low solubility in water but dissolves in some organic solvents.
Use:
Chemical intermediates: 6-methyl-3-aminopyridine is often used as an intermediate in organic synthesis for the synthesis of various compounds.
Method:
There are several ways to prepare 6-methyl-3-aminopyridine, and one of the common methods is through the reaction of ammonia sulfate and 2-methylketone-5-methylpyridine. This reaction usually needs to be carried out under alkaline conditions.
Safety Information:
It can irritate the eyes, skin, and respiratory tract, and it is necessary to avoid direct contact with the skin and eyes and ensure good ventilation when using it.
When handling this compound, measures should be taken to prevent it from polluting the environment or causing harm to human health.
When storing and transporting, the relevant laws and regulations should be observed, and they should be kept separate from combustibles, oxidants, etc. Avoid direct sunlight and high temperatures.