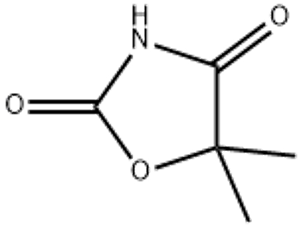
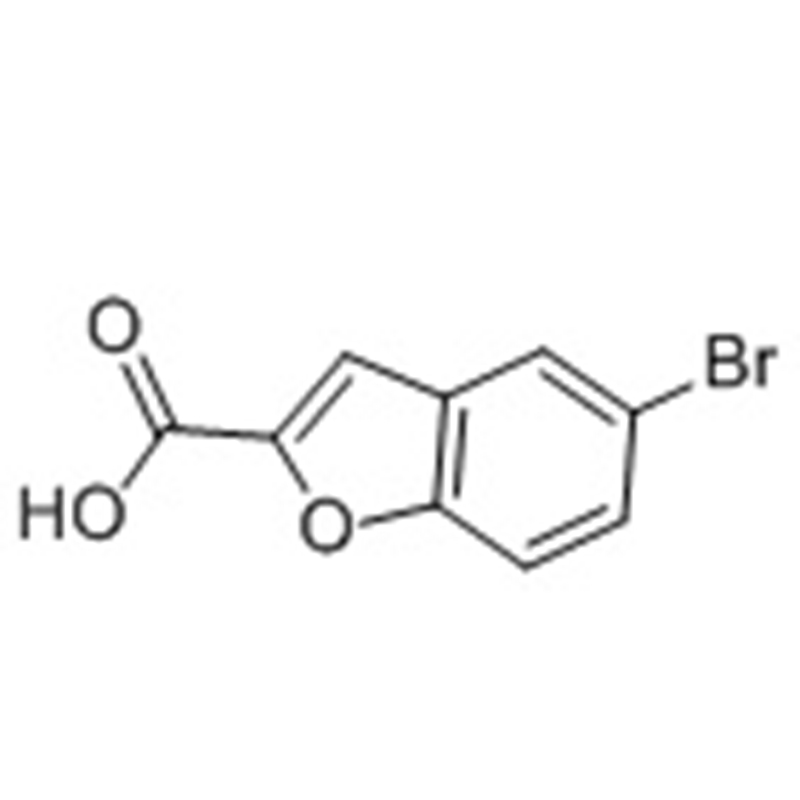
5 5-Dimethyl-1 3-oxazolidine-2 4-dione(CAS# 695-53-4)
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R40 – Limited evidence of a carcinogenic effect R33 – Danger of cumulative effects R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S22 – Do not breathe dust. S36 – Wear suitable protective clothing. S24/25 – Avoid contact with skin and eyes. S23 – Do not breathe vapour. S36/37 – Wear suitable protective clothing and gloves. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| WGK Germany | 3 |
| RTECS | RP9100000 |
| TSCA | Yes |
| HS Code | 29349990 |
| Toxicity | LD50 i.v. in mice: 450 mg/kg (Stoughton) |
Introduction
Dimethyldione is a chemical substance with the chemical name methylbenzophenone. The following is an introduction to the properties, uses, preparation methods and safety information of dimetethone:
Quality:
- Appearance: Colorless or light yellow transparent liquid.
- Solubility: Soluble in water, alcohols and ether solvents.
- Smell: With a special sweet aroma.
Use:
- Dimethyldiketone is widely used in chemical synthesis as a solvent, reducing agent and catalyst.
- It can be used as an important intermediate in organic synthesis and participates in the synthesis of a variety of organic compounds.
Method:
- The commonly used preparation method is to react benzoic acid with sulfuric acid or phosphoric acid to obtain benzoyl chloride, and then to react with methanol and sodium carbonate to obtain dimethyldione.
- There are many other ways to prepare dimethyldione, such as by chloroformic acid and phenylisocyanate reaction, by chloroazobenzene and protonated dimethylamine reaction, etc.
Safety Information:
- Dimethyldiketone is an organic compound with certain toxicity, and excessive exposure or inhalation may cause damage to the human body.
- Methadiketone should be stored in an airtight container, away from ignition and oxidants.
- Wear protective gloves and goggles when using and avoid contact with skin or eyes.
- Relevant safety operating procedures and guidelines should be followed in laboratory or industrial production processes.