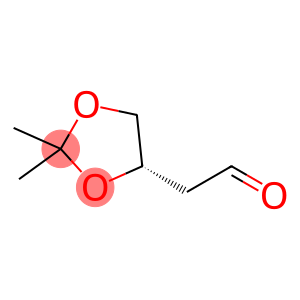
(4S)-2,2-Dimethyl-1,3-Dioxolane-4-Acetaldehyde(CAS#32233-44-6)
(4S)-2,2-Dimethyl-1,3-Dioxolane-4-Acetaldehyde(CAS#32233-44-6)
Structural features
The molecule contains a 1,3-dioxane ring structure, in which two oxygen atoms can participate in intermolecular interactions such as hydrogen bond formation, and due to the presence of the ring, the molecule has a certain rigidity and stability.
The presence of aldehyde groups (-CHO) gives the molecule typical aldehyde chemical properties, such as oxidation reaction to form carboxylic acid, reduction reaction to form alcohol, and condensation reaction with compounds containing amino groups and hydroxyl groups.
use
Organic synthesis intermediates: play an important role in the field of organic synthesis and can be used to construct a variety of complex organic compound structures. For example, by using the reactivity of aldehyde groups, it can be condensed with amine compounds to form imines, which can be further converted into functional groups and modified structures. It can also be used to introduce new carbon chains or functional groups by reacting with nucleophiles such as Grignard reagents, which provides a basis for the synthesis of organic compounds with specific structures and functions, and has been widely used in drug synthesis and total synthesis of natural products.
Chiral synthesis block: due to its chiral center, it can be used as a chiral synthesis building block for asymmetric synthesis reactions, providing chiral induction and control for the synthesis of target products with specific chiral configurations, helping to improve the optical purity and stereoselectivity of synthetic products, and is of great significance in the preparation of chiral drugs and chiral catalysts.