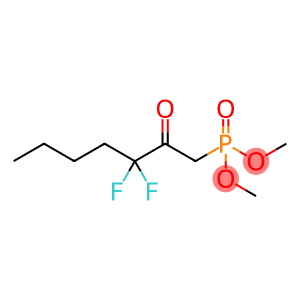
4,6-Diamino-2-mercaptopyrimidine(CAS#1004-39-3)
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| WGK Germany | 3 |
| RTECS | MB7660000 |
| HS Code | 2933 59 95 |
Introduction
4,6-Diamino-2-mercaptopyrimidine is an organic compound, often abbreviated as DAF. It has the following properties:Appearance: White crystalline solid.Solubility: Soluble in water and some organic solvents.Chemical properties: 4,6-diamino-2-mercaptopyrimidine is a reducing agent with good reducing and oxidizing properties.Its main uses:Biological research: as a fluorescent probe for the detection of aerobic reduction reactions and active argon ions, etc.Chemical analysis: used as an analytical reagent for the detection and analysis of metal ions, organic compounds, etc.Common methods for the preparation of 4,6-diamino-2-mercaptopyrimidine are as follows:Sulfhydrylation: A sulfhydryl reagent (e.g., mercaptoacetic acid) is used to react with pyrimidines to generate 4,6-diamino-2-mercaptopyrimidine.Reduction reaction: A reducing agent (e.g., hyposulfoxide) is used to reduce the compound to produce 4,6-diamino-2-mercaptopyrimidine.4,6-Diamino-2-mercaptopyrimidine is a chemical and should be followed in accordance with appropriate laboratory practices and avoid direct contact with the skin and eyes.Wear appropriate protective equipment, such as lab gloves, goggles, etc., when in use.Contact with oxygen should be avoided during storage to prevent the occurrence of oxidation reactions.When disposing of waste, it should be disposed of appropriately in accordance with local environmental regulations.


![4-[(4,6-Dihydroxy-2-pyrimidinyl)amino]benzonitrile(CAS#374067-80-8)](https://www.xinchem.com/uploads/4-46-Dihydroxy-2-pyrimidinylaminobenzonitrile.png)
![2-[(4-methoxy-3-methyl-pyridin-2-yl)methylsulfinyl]-5-pyrrol-1-yl-3H-benzoimidazole(CAS#172152-36-2)](https://www.xinchem.com/uploads/benzoimidazole.png)