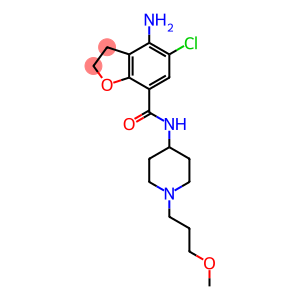
4,4-Difluorocyclohexylamine hydrochloride(CAS#675112-70-6)
| Risk Codes | 22 – Harmful if swallowed |
| Hazard Class | IRRITANT |
Introduction
4,4-Difluorocyclohexylamine hydrochloride is an organic compound with the chemical formula C6H12F2N · HCl. The following is a description of its nature, use, preparation and safety information:
Nature:
-Appearance: White or almost white crystal or crystalline powder.
-Solubility: Soluble in water, alcohol and other polar solvents, insoluble in non-polar solvents.
-Melting point: about 120-125 degrees Celsius.
Use:
- 4,4-Difluorocyclohexylamine hydrochloride can be used as an intermediate in organic synthesis and is widely used in the synthesis of organic compounds.
-It can also be used as raw materials in the fields of dyes, coatings and electronic materials.
Preparation Method:
- 4,4-Difluorocyclohexylamine hydrochloride can be prepared by the following steps:
1. hydrofluoric acid and cyanogen trichloride are reacted at an appropriate temperature to obtain 4,4-difluorocyclohexyl titanium trichloride.
2.4,4-difluorocyclohexyl titanium trichloride and ammonia water are reacted under appropriate conditions to generate 4,4-difluorocyclohexylamine.
3. react 4,4-difluorocyclohexylamine with hydrochloric acid to generate 4,4-Difluorocyclohexylamine hydrochloride.
Safety Information:
- 4,4-Difluorocyclohexylamine is hydrochloride irritant and direct contact with skin, eyes and mucous membranes should be avoided.
-Wear appropriate protective gloves, glasses and face shield during operation.
-In case of contact, rinse immediately with plenty of water and seek medical assistance.
-Store away from heat, humidity and direct sunlight.
-Avoid contact with combustibles, oxidants and strong acids to prevent chemical reactions or dangerous accidents.


![4-[(4,6-Dihydroxy-2-pyrimidinyl)amino]benzonitrile(CAS#374067-80-8)](https://www.xinchem.com/uploads/4-46-Dihydroxy-2-pyrimidinylaminobenzonitrile.png)