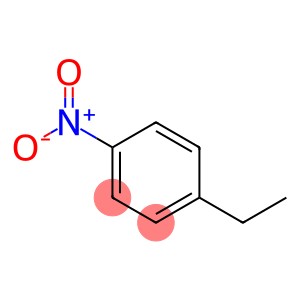
4-Nitroethylbenzene(CAS#100-12-9)
| Risk Codes | R52 – Harmful to aquatic organisms R36/37/38 – Irritating to eyes, respiratory system and skin. R33 – Danger of cumulative effects R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. |
| Safety Description | S22 – Do not breathe dust. S24/25 – Avoid contact with skin and eyes. S36 – Wear suitable protective clothing. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | 2810 |
| WGK Germany | 3 |
| RTECS | DH5600000 |
| HS Code | 29049090 |
Introduction
P-ethylnitrobenzene (abbreviation: DEN) is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of ethylnitrobenzene:
Quality:
1. Appearance: P-ethylnitrobenzene is a colorless to light yellow liquid.
2. Solubility: p-ethylnitrobenzene is soluble in alcohols and ether organic solvents.
Use:
1. Manufacture of explosives: p-ethylnitrobenzene can be used as a raw material for high-energy explosives, such as the synthesis of TNT (trinitrotoluene).
2. Detonating cord: P-ethylnitrobenzene is also used as a component of the detonating cord.
3. Chemical synthesis: p-ethylnitrobenzene is an important intermediate in organic synthesis and can be used to synthesize other compounds.
Method:
The preparation of p-ethylnitrobenzene can be used to react styrene with nitric acid to generate p-ethylaryl nitrate, and then treated with sulfuric acid to obtain p-ethylnitrobenzene.
Safety Information:
1. P-ethylnitrobenzene is a flammable liquid and should be kept away from fire and high temperatures.
2. When handling p-ethylnitrobenzene, wear protective gloves and goggles to avoid contact with skin and eyes.
3. P-ethylnitrobenzene has certain toxicity to the environment and avoids discharge into water and soil.
4. Precautions should be followed when storing and carrying p-ethylnitrobenzene.
5. When experimenting with p-ethylnitrobenzene, it should be done in a well-ventilated laboratory to avoid inhaling its vapors.