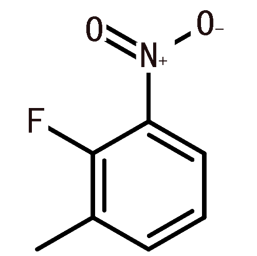
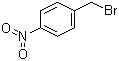
4-Nitrobenzyl bromide(CAS#100-11-8)
| Risk Codes | 34 – Causes burns |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3261 8/PG 2 |
| WGK Germany | 3 |
| RTECS | XS7967000 |
| FLUKA BRAND F CODES | 10-19-21 |
| TSCA | Yes |
| HS Code | 29049085 |
| Hazard Note | Irritant/Corrosive |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
Nitrobenzyl bromide is an organic compound, and the following is an introduction to the properties, uses, preparation methods and safety information of nitrobenzyl bromide:
Quality:
Nitrobenzyl bromide is a solid with white crystals at room temperature. It has a pungent smell and has a high melting and boiling point. The compound is insoluble in water and easily soluble in organic solvents such as ethanol and ether.
Use:
Nitrobenzyl bromide has a variety of uses in the chemical industry. It can be used as a raw material for organic synthesis reactions, and can participate in the substitution reaction of benzene ring to generate a variety of different organic compounds.
Method:
The preparation method of nitrobenzyl bromide usually involves the substitution reaction of benzene ring. A common preparation method is to use a reaction of sodium bromide (NaBr) and nitric acid (HNO3) to convert bromine to bromobenzene, which is then reacted with nitrooxides (such as nitrosobenzene or nitrosotoluene) to produce nitrobenzyl bromide.
Safety Information:
Nitrobenzyl bromide is a toxic compound that is irritating and corrosive. Contact with the skin and eyes can cause irritation and pain, and inhalation or ingestion of large amounts can cause damage to the respiratory and digestive systems. Protective gloves, glasses and masks should be worn when using nitrobenzyl bromide, and the operation should be carried out in a well-ventilated area. In addition, it should be kept away from ignition sources and oxidizers to prevent fire and explosion. Proper laboratory protocols and safety measures should be followed when handling this compound.


![5-(chloromethyl)-2 2-difluorobenzo[d][1 3]dioxole(CAS# 476473-97-9)](https://www.xinchem.com/uploads/5chloromethyl22difluorobenzod13dioxole.png)