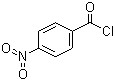
4-Nitrobenzoyl chloride(CAS#122-04-3)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
Introduction
Nitrobenzoyl chloride, chemical formula C6H4(NO2)COCl, is a pale yellow liquid with a pungent odor. The following is a description of the nature, use, preparation and safety information of nitrobenzoyl chloride:
Nature:
1. Appearance: Nitrobenzoyl chloride is a light yellow liquid.
2. smell: a pungent smell.
3. solubility: soluble in organic solvents such as ether and chlorinated hydrocarbons, slightly soluble in water.
4. Stability: relatively stable at room temperature, but will react violently with water and acid.
Use:
1. Nitrobenzoyl chloride can be used as a raw material for organic synthesis and for the preparation of other organic compounds.
2. can be used for the preparation of fluorescent dyes, dye intermediates and other chemicals.
3. Because of its high reactivity, it can be used for aromatic acyl chloride substitution reaction in organic synthesis.
Preparation Method:
The preparation of nitrobenzoyl chloride can be obtained by reacting nitrobenzoic acid with thionyl chloride in cold carbon tetrachloride, and then purifying the reaction liquid by distillation.
Safety Information:
1. Nitrobenzoyl chloride is irritating and avoid direct contact with skin and eyes.
2. use to wear protective gloves, glasses and laboratory coats and other protective equipment.
3. should be operated in a well-ventilated place to avoid inhalation of its vapor.
4. avoid violent reaction with water, acid, etc., which can cause fire or explosion.
5. Waste shall be disposed in accordance with relevant laws and regulations and shall not be discharged into the environment at will.