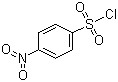
4-Nitrobenzenesulfonyl chloride(CAS#98-74-8)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R52/53 – Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 3261 8/PG 2 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 21 |
| TSCA | Yes |
| HS Code | 29049085 |
| Hazard Note | Corrosive/Moisture Sensitive |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
4-nitrobenzenesulfonyl chloride is an organic compound. Here is some information about its properties, uses, manufacturing methods and safety:
Quality:
- Appearance: 4-nitrobenzenesulfonyl chloride is a colorless to pale yellow crystalline or crystalline solid.
- Flammability: 4-nitrobenzenesulfonyl chloride can burn when exposed to open flames or high temperatures, releasing toxic fumes and gases.
Use:
- Chemical intermediates: It is often used as an important raw material or intermediate in organic synthesis for the preparation of other organic compounds.
- Research uses: 4-nitrobenzenesulfonyl chloride can also be used in certain reactions and reagents in chemical research or experiments.
Method:
- The preparation method of 4-nitrobenzene sulfonyl chloride generally adopts nitro substitution reaction. It is usually obtained by reacting 4-nitrobenzene sulfonic acid with thionyl chloride.
Safety Information:
- Irritating effect on skin and eyes: Exposure to 4-nitrobenzenesulfonyl chloride may cause skin inflammation, eye irritation, etc.
- Toxic: 4-nitrobenzenesulfonyl chloride is toxic and should be avoided for ingestion or inhalation.
- May react dangerously with other substances: This substance may react dangerously with combustibles, strong oxidants, etc., and should be stored separately from other substances.