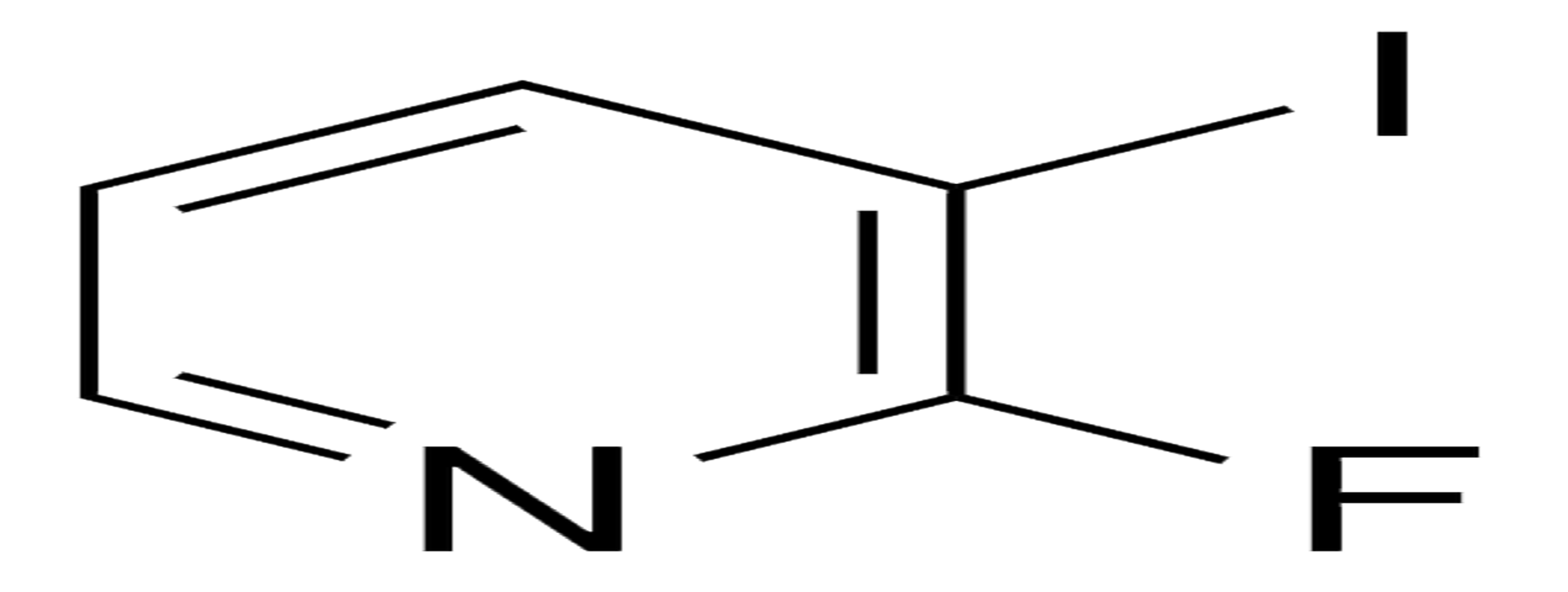
4-Methoxyphenylhydrazine hydrochloride(CAS# 19501-58-7)
Risk and Safety
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S36/37 – Wear suitable protective clothing and gloves. S36 – Wear suitable protective clothing. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | 2811 |
| WGK Germany | 3 |
| HS Code | 29280090 |
| Hazard Note | Irritant/Harmful |
| Hazard Class | IRRITANT, KEEP COLD |
| Packing Group | III |
4-Methoxyphenylhydrazine hydrochloride(CAS# 19501-58-7) Information
| Use | 4-methoxyphenylhydrazine hydrochloride is an intermediate, mainly used to produce phenylhydrazine compounds, and can also be used to produce other chemical products, such as 4-nitroindole and apixaban. Applied to dyes and pharmaceutical intermediates |
| Preparation | 4-methoxyphenylhydrazine hydrochloride can be prepared from aniline through diazotization reaction. Take aniline, hydrochloric acid and sodium nitrite, the molar ratio between them is 1: 3.2: 1.0, first add hydrochloric acid, then add ammonium nitrite at 5 ℃, and react at 0~20 ℃ for 40 minutes to generate chlorinated diazobenzene; According to the molar ratio of aniline to 1: 3.5: 2.5, ammonium sulfite and hydrochloric acid are added, and reduction, hydrolysis and acidification are carried out in the reduction kettle, the reduction time is 60~70 minutes, and the hydrolysis and acidification time is 50 minutes. First, ammonium sulfite reacts with excess hydrochloric acid to produce ammonium bisulfite, ammonium bisulfite, ammonium sulfite reacts with chlorinated diazobenzene to form phenylhydrazine disulfonate, and then reacts with hydrochloric acid for hydrolysis and acid analysis The reaction, and after spin-drying, 4-methoxyphenylhydrazine hydrochloride is prepared. |
Write your message here and send it to us



![2-Amino-6-bromothiazolo[5,4-b]pyridine(CAS#1160791-13-8)](https://www.xinchem.com/uploads/bromothiazolopyridine.png)