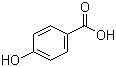
4-Hydroxybenzoic acid(CAS#99-96-7)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S37/39 – Wear suitable gloves and eye/face protection |
4-Hydroxybenzoic acid(CAS#99-96-7)introduce
Hydroxybenzoic acid, also known as p-hydroxybenzoic acid, is an organic compound.
Its main properties are as follows:
Physical properties: Hydroxybenzoic acid is a white or slightly yellow crystal with a unique aromatic odor.
Chemical properties: Hydroxybenzoic acid is slightly soluble in water and soluble in alcohols. It is an acidic carboxylic acid that can form salts with metals. It can also react with aldehydes or ketones, undergo condensation reactions, and form ether compounds.
Reactivity: Hydroxybenzoic acid can undergo neutralization reaction with alkali to form benzoate salt. It can participate in esterification reaction under acid catalysis to generate p-hydroxybenzoate ester. Hydroxybenzoic acid is also an intermediate of plant growth regulators.
Application: Hydroxybenzoic acid can be used to synthesize plant growth regulators, dyes, fragrances, and other chemicals.