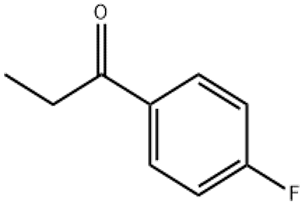
4′-Fluoropropiophenone(CAS# 456-03-1)
| Risk Codes | R22 – Harmful if swallowed R37/38 – Irritating to respiratory system and skin. R41 – Risk of serious damage to eyes R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S39 – Wear eye / face protection. S37/39 – Wear suitable gloves and eye/face protection |
| UN IDs | 2735 |
| WGK Germany | 2 |
| HS Code | 29147000 |
| Hazard Note | Irritant |
Introduction
Fluoropropionone (also known as benzene 1-fluoroacetone) is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of fluoropropionone:
Quality:
Appearance: Fluoropropion is a colorless liquid with a strong pungent odor.
Density: The density of fluoropropion is about 1.09 g/cm³.
Solubility: It is soluble in organic solvents such as ethanol, ether and acetone, but insoluble in water.
Reactivity: It can react with a reducing agent to produce the corresponding alcohol compounds. Fluoropropiophenone can undergo explosive reactions under the action of oxidizing agents.
Use:
Fluoropropiophenone has certain uses, mainly including:
As an organic synthesis reagent: Fluoropropion can be used as a ligand or participate in more complex organic reactions, such as fluorination and acylation.
As a surfactant: due to its special structure and properties, it has application potential in wetting, decontamination and emulsification.
Method:
Fluoropylacetone can be prepared by the reaction of fluorinated acetone and benzene, generally under the condition of adding a fluorinating agent catalyst such as boron trifluoride (BF3) or aluminum fluoride (AlF3) in an inert atmosphere.
Safety Information:
Fluoropropion is irritating and may cause irritation and burns in contact with the skin and eyes. Appropriate precautions, such as gloves, goggles, and protective clothing, should be taken during contact.
It is combustible and should be kept away from open flames and high temperature sources. When handling and storing, fire prevention measures should be taken.
When used in laboratories and industries, proper operating procedures should be followed to avoid unsafe reactions with other hazardous substances.
Fluoropionone should be stored in a cool, dry and well-ventilated place, away from fire and oxidants.