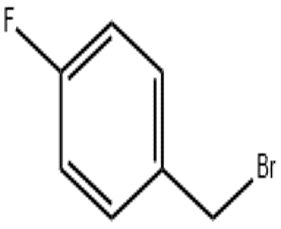
4-Fluorobenzyl bromide(CAS# 459-46-1)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R36 – Irritating to the eyes |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29039990 |
| Hazard Note | Corrosive/Lachrymatory |
| Hazard Class | 8 |
| Packing Group | III |
Introduction
Fluorobenzyl bromide is an organic compound. It is a colorless to pale yellow solid with a strong aromatic odor.
Fluorobenzyl bromide has many important properties and uses. It is an important intermediate widely used in the field of organic synthesis. Fluorobenzyl bromide can introduce functional groups with special chemical activity into the aromatic ring through substitution reactions, and is also commonly used in the preparation of functional compounds.
A common method for the preparation of fluorobenzyl bromide is to react benzyl bromide with anhydrous hydrofluoric acid. In this reaction, hydrofluoric acid acts as a bromine atom and introduces a fluorine atom.
It is an organic substance that has a certain toxicity. May cause irritation and damage to the skin, eyes, and respiratory system. Appropriate protective equipment such as gloves, goggles, and protective masks need to be worn during operation. Prolonged exposure to the vapors of flubromide should be avoided to avoid poisoning. If you accidentally come into contact with fluorobenzyl bromide or its vapors, you should immediately rinse with clean water and seek medical attention in time. When storing fluorobenzyl bromide, it should be placed in a fire-resistant, well-ventilated and airtight container, away from ignition and other flammable materials.