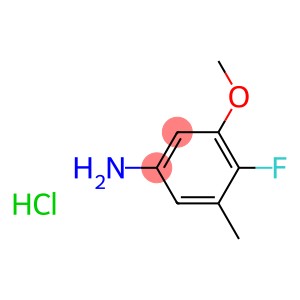
“4-Fluoro-3-methoxy-5-methylaniline Hydrochloride(CAS#1357103-70-8)”
4-Fluoro-3-methoxy-5-methylaniline Hydrochloride(CAS#1357103-70-8)
Physicochemical properties
Appearance: Usually white or off-white crystalline powder, but the actual appearance may vary slightly depending on the synthesis process and purity.
Solubility: As hydrochloride, it has good solubility in water and can ionize the corresponding ions. It also has a certain solubility in some polar organic solvents such as ethanol and methanol, but has poor solubility in non-polar organic solvents such as n-hexane and ether.
Stability: It is relatively stable under dry conditions at room temperature, but chemical reactions may occur at high temperature, high humidity or when in contact with strong oxidants, strong alkalis, etc. For example, under alkaline conditions, a dehydrochloric acid reaction may occur to regenerate free amines.
Synthesis method
Generally, 4-fluoro-3-methoxy-5-methylnitrobenzene can be used as the starting material, and the nitro group can be converted into an amino group through a reduction reaction to obtain 4-fluoro-3-methoxy-5-methylaniline, and then reacted with hydrochloric acid into a salt to obtain the target product 4-fluoro-3-methoxy-5-methylaniline hydrochloride. The reduction reaction can be carried out in a hydrogen atmosphere using catalysts such as palladium and carbon. Chemical reduction methods can also be used, such as iron powder, zinc powder, etc. for reduction under acidic conditions.
Fields of application
Pharmaceutical field: It is an important pharmaceutical intermediate, which can be used to synthesize a variety of biologically active drug molecules. For example, it can be used as a building block for the synthesis of some antimicrobial drugs, nervous system drugs, etc., and its unique fluorine atom, methoxy and methyl group and other functional groups can affect the binding ability and pharmacokinetic properties of drug molecules to targets.
In the field of materials science, it can be used to synthesize functional materials with special properties, such as in organic optoelectronic materials, the aromatic amine part of its structure can provide good electron transport properties, and fluorine atoms can adjust the optical and electrical properties of materials, so as to be used to prepare organic light-emitting diodes (OLEDs), organic solar cells and other materials.