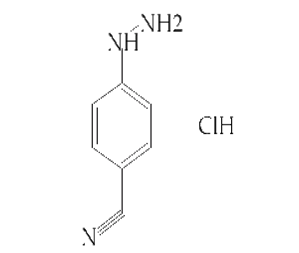
4-Cyanophenylhydrazine hydrochloride(CAS# 2863-98-1)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| UN IDs | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| HS Code | 29280000 |
| Hazard Note | Harmful |
| Packing Group | III |
Introduction
4-Cyanophenylhydrazine hydrochloride is an organic compound with the chemical formula C6H6N4 · HCl. The following is an introduction to its nature, use, preparation and safety information:
Nature:
4-Cyanophenylhydrazine hydrochloride is a white crystalline solid, soluble in water and some organic solvents. It is flammable and may produce toxic gases.
Use:
4-Cyanophenylhydrazine hydrochloride is a commonly used intermediate compound. It can be used as a raw material for organic synthesis reactions, for example, for the synthesis of dyes, fluorescent dyes or organometallic complexes, etc. In addition, it is also used in the pharmaceutical field as a synthetic intermediate for certain drugs.
Method:
4-Cyanophenylhydrazine hydrochloride is generally prepared by reacting phenylhydrazine hydrochloride with sodium cyanide. Phenylhydrazine hydrochloride and sodium cyanide are first dissolved in the corresponding solvent, then the two solutions are mixed and the reaction is stirred at an appropriate temperature for a period of time. Finally, the crude product is obtained by filtration, and purified by washing and recrystallization to obtain the pure product.
Safety Information:
4-Cyanophenylhydrazine hydrochloride is irritating and corrosive and may cause damage to the skin, eyes and respiratory tract. Appropriate protective equipment such as gloves, goggles and masks should be worn during use. Avoid dust during operation and maintain a well-ventilated laboratory environment. If you accidentally come into contact with it, you should immediately rinse with plenty of water and seek medical treatment. In addition, it should be kept away from fire and oxidizing agents and stored in a dry, cool place.