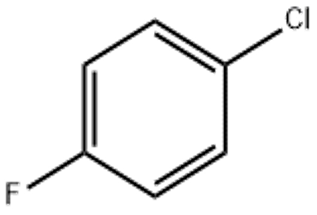
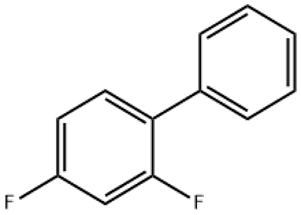
4-Chlorofluorobenzene(CAS# 352-33-0)
| Risk Codes | R10 – Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. R39/23/24/25 - R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R11 – Highly Flammable |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S37/39 – Wear suitable gloves and eye/face protection S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37 – Wear suitable protective clothing and gloves. S7 – Keep container tightly closed. |
| UN IDs | UN 1993 3/PG 3 |
| WGK Germany | 3 |
| TSCA | T |
| HS Code | 29039990 |
| Hazard Note | Flammable/Irritant |
| Hazard Class | 3 |
| Packing Group | III |
Introduction
Chlorofluorobenzene is an organic compound. It is a colorless liquid with an off-odor. The following is an introduction to the nature, use, preparation method and safety information of chlorofluorobenzene:
Quality:
Chlorofluorobenzene has unique physicochemical properties, solubility and volatility. At room temperature, it is stable, but can be reacted with strong oxidants and strong reducing agents. The chlorine and fluorine atoms in its molecule, chlorofluorobenzene has certain reactivity.
Use:
Chlorofluorobenzene has a variety of uses in industry. Chlorofluorobenzene can also be used as a solvent in the synthesis of organometallic compounds and inks.
Method:
The preparation of chlorofluorobenzene is usually obtained by the reaction of chlorobenzene with hydrogen fluoride. This reaction needs to be carried out in the presence of catalysts, such as zinc fluoride and iron fluoride. The reaction conditions are generally carried out at high temperatures, with a common temperature of 150-200 degrees Celsius.
Safety information: Chlorofluorobenzene is irritating to the skin and eyes, and direct contact should be avoided when touched. During operation, good ventilation measures should be taken to avoid inhalation of the substance. Chlorofluorobenzene is a combustible substance and should be avoided from contact with ignition sources and high temperature environments. When storing, it should be placed in a cool, dry and well-ventilated place, away from fire and oxidants.