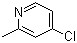
4-Chloro-2-methylpyridine(CAS#3678-63-5)
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S23 – Do not breathe vapour. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S37/39 – Wear suitable gloves and eye/face protection |
Introduction
4-Chloro-2-picoline is an organic compound with the chemical formula C7H4ClN having a chlorine atom and a methyl group substituted on the pyridine ring at position 2.
The compound is a colorless liquid with a unique aroma. It is soluble in most organic solvents, but poorly soluble in water.
In practical applications, 4-Chloro-2-picoline is widely used in organic synthesis and pharmaceutical research. It can be used as an effective catalyst to participate in a variety of organic reactions, such as oxidation, reduction, alkylation and condensation reactions. In addition, it can also be used as the synthesis of pharmaceutical intermediates and the production of pesticides.
4-Chloro-2-picoline is prepared by chlorination of pyridine. The specific method is to react 2-methylpyridine with chlorine gas or hypochlorous acid to generate 4-Chloro-2-picoline.
Regarding safety information, 4-Chloro-2-picoline needs to comply with general laboratory safety measures during use. It is an organic solvent, which is volatile and must be used in a well-ventilated environment. Avoid contact with skin, eyes and respiratory tract and use appropriate personal protective equipment when necessary. Reactions with strong oxidants and strong acids should be avoided during storage and handling. Personal protective measures and waste disposal should be carried out if necessary. Observe local environmental regulations during handling or disposal.