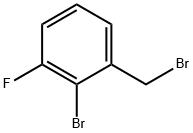
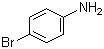
4-Bromoaniline(CAS#106-40-1)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R21/22 – Harmful in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37 – Wear suitable protective clothing and gloves. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| UN IDs | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | BW9280000 |
| FLUKA BRAND F CODES | 8-9-23 |
| TSCA | Yes |
| HS Code | 29214210 |
| Hazard Note | Harmful |
| Hazard Class | 6.1 |
| Packing Group | III |
| Toxicity | LD50 orally in Rabbit: 456 mg/kg LD50 dermal Rat 536 mg/kg |
Introduction
Bromoaniline is an organic compound. The following is an introduction to its nature, use, manufacturing methods and safety information:
Quality:
- Appearance: Bromoaniline is a colorless to yellowish solid.
- Solubility: It is not easily soluble in water, but it is soluble in many organic solvents.
Use:
- Bromoaniline is mainly used in organic synthesis reactions and can be used as a starting material or intermediate in organic synthesis.
- In some cases, bromoaniline is also used as a reagent for silver mirror reactions.
Method:
- The preparation of bromoaniline is usually obtained by the reaction of aniline with hydrogen bromide. During the reaction, aniline and hydrogen bromide undergo an aminolysis reaction to produce bromoaniline.
- This reaction can be carried out in an anhydrous alcohol solution, such as in ethanol or isopropanol.
Safety Information:
- Bromoaniline is a corrosive substance and should be protected from contact with the skin, eyes and respiratory tract.
- Wear appropriate personal protective equipment such as protective gloves, glasses, and respirators when in use.
- Avoid contact with oxidants and strong acids to prevent possible dangerous reactions.
- When storing and handling, avoid mixing with other chemicals to avoid accidents.
When operating, the relevant chemical laboratory safety practices and operating guidelines must be followed.


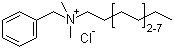
![4-methyl-1-oxaspiro[5.5]undecene(CAS#68228-06-8)](https://www.xinchem.com/uploads/4-methyl-1-oxaspiro5.5undecene.jpg)