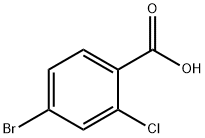
4-Bromo-2-chlorobenzoic acid(CAS# 59748-90-2)
| Risk Codes | R22 – Harmful if swallowed R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
| Safety Description | S60 – This material and its container must be disposed of as hazardous waste. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| HS Code | 29163990 |
| Hazard Class | IRRITANT |
| Packing Group | Ⅲ |
Introduction
Quality:
2-Chloro-4-bromobenzoic acid is a solid with a white crystalline appearance. It has good solubility at room temperature and can be dissolved in some common organic solvents, such as ethanol and ether.
Use:
2-Chloro-4-bromobenzoic acid can be used as an intermediate in organic synthesis. It can also be used in the preparation of organic light-emitting diodes (OLEDs) as one of the important materials in this field.
Method:
2-Chloro-4-bromobenzoic acid is prepared in a variety of ways, and benzoic acid is often used as a starting material in the laboratory. Specific synthesis methods include reactions such as chlorination, bromination, and carboxylation, which usually require the use of catalysts and reagents.
Safety Information:
2-Chloro-4-bromobenzoic acid is an organic compound, and for safety reasons, appropriate personal protective equipment such as gloves, glasses, and lab clothing should be worn during handling. It can cause irritation to the eyes, skin, and respiratory tract and needs to be avoided. It should be kept away from open flames and high temperatures when it is stored and used to avoid the production of toxic gases.