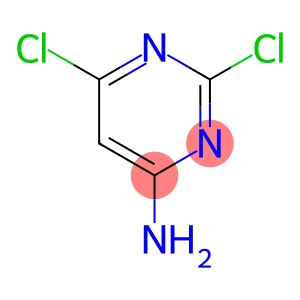
4-Amino-2,6-dichloropyrimidine(CAS#10132-07-7)
| Risk Codes | R22 – Harmful if swallowed R36/37/38 – Irritating to eyes, respiratory system and skin. R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37 – Wear suitable protective clothing and gloves. S36 – Wear suitable protective clothing. |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29333999 |
| Hazard Note | Irritant |
Introduction
4-Amino-2,6-dichloropyrimidine is an organic compound. The following is a detailed introduction to the properties, uses, preparation methods and safety information of this compound:Quality:- Appearance: 4-Amino-2,6-dichloropyrimidine is a colorless to light yellow solid.- Solubility: It is less soluble in water, but it can be better soluble in organic solvents.- Chemical reactions: Under acidic conditions, 4-amino-2,6-dichloropyrimidine can react with other compounds, such as dyes that form a green color with amines.Use:Method:- The preparation method of 4-amino-2,6-dichloropyrimidine can be obtained by reacting 2,6-dichloropyrimidine with ammonia. 2,6-dichloropyrimidine was dissolved in ethanol, ammonia was added, and the reaction was carried out for a period of time. Then, through steps such as filtration, cooling, and crystallization, the target product can be obtained.Safety Information:- 4-Amino-2,6-dichloropyrimidine has certain toxicity and should be handled and used under suitable laboratory conditions.- The compound should be stored and handled in a way that avoids contact with strong acids and strong oxidants to prevent dangerous reactions.