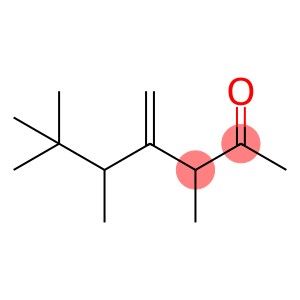
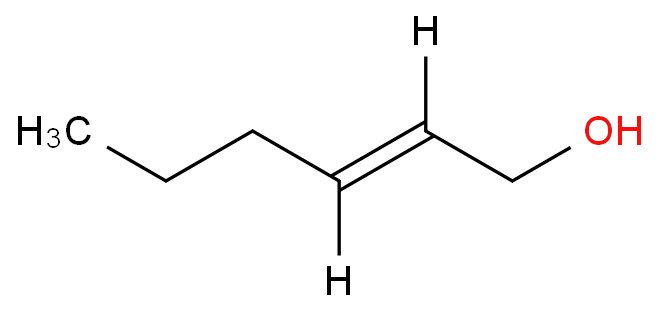
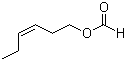3,5,6,6-tetramethyl-4-methyleneheptan-2-one(CAS#81786-75-6/2020341-69-7/81786-73-4/81786-74-5/86115-11-9)
3,5,6,6-tetramethyl-4-methyleneheptan-2-one(CAS#81786-75-6/2020341-69-7/81786-73-4/81786-74-5/86115-11-9)
Physical Appearance: Usually it may be a colorless to pale yellow clear liquid, but the exact appearance may vary slightly due to factors such as purity.
Odor: May have a special smell, similar to the smell of certain ketone compounds, but the specific smell needs to be determined by actual smell and may vary depending on the individual’s sense of smell.
Solubility: Generally soluble in common organic solvents, such as ethanol, ether, benzene, etc., which is related to its organic molecular structure and follows the principle of similar solubility. However, it is less soluble in water because its molecules are more hydrophobic as a whole.
Boiling and melting points: Accurate boiling and melting point data should be determined experimentally or by consulting professional literature. However, it is roughly speculated that the boiling point may be relatively high due to the fact that a certain number of methyl groups in its molecule increase the intermolecular forces; The melting point is related to the symmetry and regularity of the molecule, and the structural characteristics of the compound will affect its melting point. Chemical properties Stability: It has a certain stability under normal temperature and pressure and general storage conditions. However, chemical reactions may occur under special conditions such as high temperature, strong acid, strong alkali or strong oxidant. For example, the keto carbonyl group may undergo nucleophilic addition reactions under acidic or alkaline conditions, etc.
Reactivity: The presence of methylene groups gives the compound a certain reactivity. It can participate in some reactions related to carbon-carbon double bonds, such as addition reactions, and additions occur with reagents such as halogens, hydrogen halides, etc. At the same time, the ketone carbonyl group can also undergo a series of reactions, such as reacting with alcohols to form ketone.
Use Fragrance: Its unique smell makes it possible to use it as a fragrance ingredient in the fragrance industry, adding unique aroma components to perfumes, essences and other products, which can be used to blend notes with a special style. Organic synthesis intermediates: due to a variety of functional groups in their structure, they are important intermediates in organic synthesis. Various organic compounds with specific functions can be prepared by different chemical reactions, which can be used in many fields such as drug synthesis and materials science. For example, the reactivity of its ketone carbonyl and methylene groups can be used to construct more complex molecular structures.