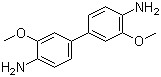
3,3′-Dimethoxybenzidine(CAS#119-90-4)
| Hazard Symbols | T – Toxic |
| Risk Codes | R45 – May cause cancer R22 – Harmful if swallowed |
| Safety Description | S53 – Avoid exposure – obtain special instructions before use. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | 2811 |
| WGK Germany | 3 |
| RTECS | DD0875000 |
| TSCA | Yes |
| HS Code | 29222990 |
| Hazard Class | 6.1(a) |
| Packing Group | II |
| Toxicity | Dianisidine is a probable carcinogen used in the manufacture of dyes. EPA has classified as a Group 2B—probable human carcinogen. |
Introduction
Dimethoxyaniline (N-methylaniline) is an organic compound. It is an organic amine with an alcohol-amine nature and a pKa of about 4.64. The following is an introduction to the properties, uses, preparation methods and safety information of dimethoxyaniline:
Quality:
- Appearance: Dimethoxyaniline is a colorless to light yellow liquid.
- Solubility: It can be dissolved in many organic solvents, such as alcohols, ethers, and chlorinated hydrocarbons.
- Toxicity: It is a toxic substance, and exposure to or inhalation of high concentrations of vapors or liquids can be harmful to health.
Use:
- Dimethoxyaniline is mainly used as an intermediate in organic synthesis.
- It can also be used as a catalyst to be added to the reaction system to facilitate certain chemical reactions.
- The reactivity of dimethoxyaniline with other compounds, its reaction with carbamate and amide compounds becomes an important step in the synthesis of new compounds.
Method:
- Dimethoxyaniline can be prepared by the reaction of aniline and methanol. Reactions under acidic conditions, such as the use of hydrochloric acid or sulfuric acid as catalysts, can facilitate the reaction.
Safety Information:
- Lemonaniline is irritating to the skin and eyes and potentially dangerous to the respiratory and digestive systems.
- Appropriate precautions such as wearing protective gloves, goggles, and protective clothing are required when using dimethoxyaniline to ensure well-ventilated experimental conditions.
- When storing and handling bimethoxyaniline, avoid contact with strong oxidants and flammable materials, and store in a cool, ventilated and dry place.