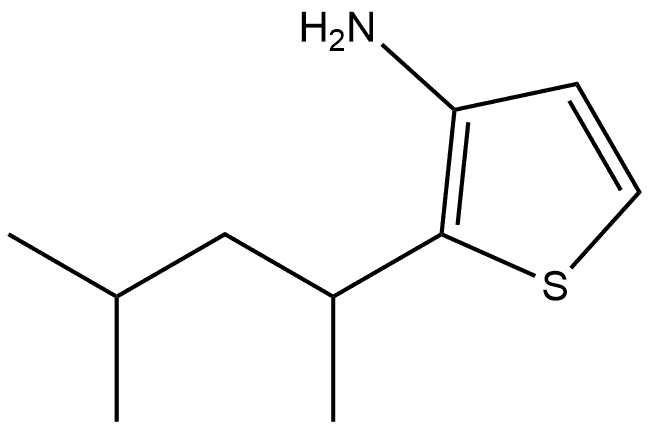
3-Thiophenamine, 2-(1,3-dimethylbutyl)-(CAS#183677-34-1)
3-Thiophenamine, 2-(1,3-dimethylbutyl)-(CAS#183677-34-1)
Appearance: May be colorless to light yellow liquid or solid, depending on its purity and environmental conditions.
Solubility: Due to the presence of thiophene rings and amino groups in the molecule, it may have good solubility in some organic solvents such as ethanol, methanol, dichloromethane, etc., and may have poor solubility in water.
Stability: It is relatively stable at room temperature and pressure, but under the conditions of high temperature, strong acid, strong alkali or strong oxidant, chemical reactions may occur, such as amino groups may be protonated, and thiophene rings may undergo electrophilic substitution and other reactions.
Structural features
Contains thiophene ring, thiophene is an aromatic five-membered heterocyclic compound, and the lone pair of electrons on its sulfur atom participates in the conjugation of the aromatic system, so that the thiophene ring has a certain stability and special electron cloud distribution, and can undergo various typical aromatic compound reactions, such as electrophilic substitution reaction.
There is a 1,3-dimethylbutyl group attached to the 2nd position, and this larger alkyl chain affects the physical and chemical properties of the molecule, such as increasing the fat solubility of the molecule, which makes the solubility of the molecule in non-polar solvents enhanced. At the same time, due to the electron donor of the alkyl group, it will affect the electron cloud density distribution on the thiophene ring, which may increase the electron cloud density adjacent to the amino group on the thiophene ring, thereby affecting its reactivity.
The 3rd position is an amino group (-NH₂), which is a strong electron donor group, which can increase the electron cloud density of the thiophene ring, especially the ortho and para-electron cloud density of the amino group is more obvious, which makes the molecule have high activity in the electrophilic substitution reaction, and the amino group can also participate in a variety of chemical reactions, such as reacting with acid to form salts, and reacting with acid chloride and acid anhydride to form amides.