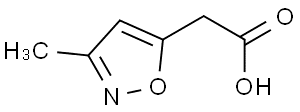
3-METHYL-5-ISOXAZOLEACETIC ACID(CAS#19668-85-0 )
Risk and Safety
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. |
| WGK Germany | 3 |
| HS Code | 29349990 |
3-METHYL-5-ISOXAZOLEACETIC ACID(CAS#19668-85-0 ) Introduction
-Appearance: White crystalline solid
-Melting Point: 157-160 ℃
-relative molecular mass: 141.13g/mol
-Solubility: Slightly soluble in water, soluble in alcohol, ether and organic solvents
-Chemical properties: 3-methyll-5-isoxazoleacetic ACID can be acylated, carbonylated and substituted by ACID-catalyzed reactions.
Use:
-Pharmaceutical field: 3-METHYL-5-ISOXAZOLEACETIC ACID is used as a synthetic intermediate and is commonly used in the preparation of drugs and biologically active molecules.
-Pesticide field: It can also be used as a raw material for pesticides, used to prepare pesticides, fungicides and herbicides.
Method:
The preparation method of 3-methyll-5-isoxazoleacetic ACID is more complicated, but it can be carried out through the following steps:
1. First prepare 5-Isoxazolylmethanol (5-Isoxazolylmethanol).
2. Using pyruvic acid (Acetone) and Potassium nitrate (Potassium nitrate) in the presence of iodide ions for nitration reaction, preparation of 5-Isoxazolylcarboxylic acid (5-Isoxazolylcarboxylic acid).
3. Acylation of 5-isoxazolyl carboxylic ACID using methanol and sulfuric ACID to generate 3-METHYL-5-ISOXAZOLEACETIC ACID.
Safety Information:
When handling 3-methyll-5-isoxazoleacetic ACID, the following safety precautions should be taken:
-Avoid contact with skin and eyes. If contact occurs, rinse immediately with plenty of water.
-Wear appropriate personal protective equipment such as goggles, gloves and lab coats.
-Avoid inhaling its vapor or dust, and provide adequate ventilation during operation.
-When performing laboratory-scale preparations, follow the safe practices of the chemical laboratory.


![3-[(3-amino-4-methylamino-benzoyl)pyridin-2-yl-amino]-(CAS# 212322-56-0)](https://www.xinchem.com/uploads/33amino4methylaminobenzoylpyridin2ylamino.png)