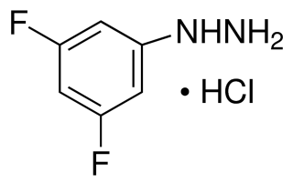
3 5-Difluorophenylhydrazine hydrochloride(CAS# 502496-27-7)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. |
| WGK Germany | 3 |
| HS Code | 29280000 |
| Hazard Note | Irritant |
Introduction
3,5-difluorophenylhydrazine hydrochloride is an organic compound. The following is an introduction to its nature, use, manufacturing method and safety information:
Properties: It is soluble in water and some organic solvents such as ethanol, methanol. It is a weakly acidic substance that reacts with alkalis.
Use:
3,5-difluorophenylhydrazine hydrochloride is often used as a reducing agent and activator in organic synthesis. It can be used for addition reactions, reducing organic compounds such as ketones, aldehydes, aromatic ketones, etc.
Method:
3,5-Difluorophenylhydrazine hydrochloride can be obtained by the reaction of hydroquinone and 2-chloro-1,3,5-trifluorobenzene. In general, hydroquinone reacts with excess 2-chloro-1,3,5-trifluorobenzene under alkaline conditions to obtain 3,5-difluorophenylhydrazine. By reacting it with hydrogen chloride, 3,5-difluorophenylhydrazine hydrochloride can be obtained.
Safety Information:
3,5-Difluorophenylhydrazine hydrochloride is a chemical that is generally used in laboratories and industrial production. Proper protocols should be followed during the procedure, and appropriate personal protective equipment such as gloves, safety glasses, and lab coats should be worn. It is less toxic, but it should still be avoided from contact with the skin, eyes, and inhalation. In case of exposure, it is necessary to rinse quickly with plenty of water and seek medical attention promptly. During storage, it should be kept away from fire sources and flammable materials, and stored in a dry, well-ventilated place.