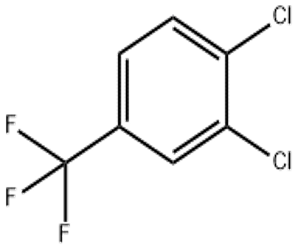
3 4-Dichlorobenzotrifluoride(CAS# 328-84-7)
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R34 – Causes burns R22 – Harmful if swallowed |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S60 – This material and its container must be disposed of as hazardous waste. S20 – When using, do not eat or drink. |
| UN IDs | 1760 |
| WGK Germany | 2 |
| RTECS | CZ5527510 |
| TSCA | Yes |
| HS Code | 29036990 |
| Hazard Note | Irritant |
| Hazard Class | 8 |
| Packing Group | III |
Introduction
3,4-Dichlorotrifluorotoluene (also known as 3,4-dichlorotrifluoromethylbenzene) is an organic compound.
3,4-Dichlorotrifluorotoluene is a colorless liquid and insoluble in water. Its main characteristics are high chemical stability and strong solvency. Its special structure, it has good thermal stability at high temperatures.
In practical applications, 3,4-dichlorotrifluorotoluene is used as an intermediate in organic synthesis. In addition, it can be used as a surfactant and solvent.
The method for preparing 3,4-dichlorotrifluorotoluene is mainly obtained by fluorination and chlorination of trifluorotoluene. This process usually takes place in an inert gas atmosphere and requires the use of reactants and catalysts.
Write your message here and send it to us



![5H-Pyrrolo[3,2-d]pyrimidine,7-bromo-4-methoxy-5-[(phenylmethoxy)methyl](CAS#299916-62-4)](https://www.xinchem.com/uploads/pyrimidinebromomethoxyphenylmethoxymethyl.png)