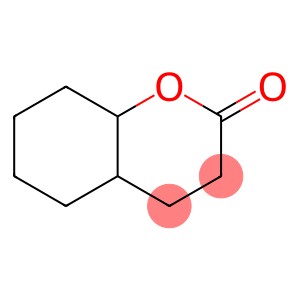
(2,6,6-Trimethyl-2-hydroxycyclohexylidene)acetic acid lactone(CAS#17092-92-1)
| Safety Description | 24/25 – Avoid contact with skin and eyes. |
(2,6,6-Trimethyl-2-hydroxycyclohexylidene)acetic acid lactone(CAS#17092-92-1)
1. Basic Information
Name: (2,6,6-Trimethyl-2-hydroxycyclohexylidene) acetic acid lactone.
CAS number: 17092-92-1, which is the unique identification number of the compound in the chemical substance registration system, which is convenient for accurate query and data retrieval worldwide.
Second, structural characteristics
Its molecular structure contains a six-membered cyclohexyl group with a hydroxyl group attached to the 2 position, and a trimethyl substituent at this position, which gives the molecule a certain steric hindrance and electronic properties. There is also a lactone structure formed by methylene group and carbonyl group in the molecule, which has certain stability and has a key impact on the chemical activity, solubility and other physical and chemical properties of the compound.
3. Physical properties
Appearance: Usually white to light yellow crystalline powder or solid, relatively stable state, easy to store and handle.
Solubility: It has a certain solubility in common organic solvents such as ethanol, ether, chloroform, etc., and can form a uniform solution for subsequent chemical reactions or analytical tests; It has poor solubility in water and follows the principle of “similar dissolvability”, reflecting its non-polar molecular nature.
Melting point: It has a relatively fixed melting point range, which is one of the important indicators of purity identification, and the purity of the sample can be preliminarily judged by accurately determining the melting point, and the specific melting point value can be consulted in professional chemical literature or databases.
Fourth, chemical properties
It has the typical ring-opening and closed-loop reactivity of lactone, and under the catalytic conditions of acid and alkali, the lactone ring can be broken, and it reacts with nucleophiles and electrophiles to generate a series of derivatives, providing a variety of paths for organic synthesis.
As an active functional group, hydroxyl group can participate in esterification, etherification and other reactions to further modify the molecular structure and expand its application range, such as the preparation of ester compounds with special biological activity for drug research and development.
5. Synthesis method
A common synthetic route is to use cyclohexanone derivatives with suitable substituents as the starting material, and construct the target molecular structure through multi-step reactions. For example, trimethyl groups are introduced through alkylation reaction, and then lactone rings and hydroxyl groups are constructed by oxidation and cyclization, and the reaction conditions such as temperature, pH, reaction time, etc. need to be strictly controlled throughout the process to ensure high yield and purity.
Sixth, the field of application
Fragrance industry: due to its unique structure brings special odor, it can be used as a flavor ingredient in perfumes, cosmetics, food fragrance additives, etc., after dilution and blending, to add unique flavor.
Pharmaceutical field: As an intermediate in drug synthesis, its structural fragments can be introduced into molecules with pharmacological activity to modify activity, improve pharmacokinetic properties, and help the research and development of new drugs, which is expected to be used for the treatment of a variety of diseases.
Organic synthesis: As a key building block, it participates in the construction of the total synthesis of complex natural products and the preparation of new organic functional materials, promotes the development of the field of organic chemistry, and provides a basis for the creation of new substances.


![5H-Pyrrolo[3,2-d]pyrimidine,7-bromo-4-methoxy-5-[(phenylmethoxy)methyl](CAS#299916-62-4)](https://www.xinchem.com/uploads/pyrimidinebromomethoxyphenylmethoxymethyl.png)