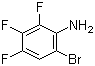
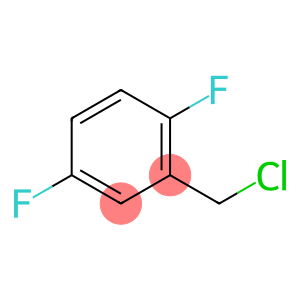
2,5-Difluorobenzyl chloride(CAS#495-07-8)
| Risk Codes | R34 – Causes burns R20/22 – Harmful by inhalation and if swallowed. |
| Safety Description | S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | UN1760 |
| HS Code | 29039990 |
| Hazard Note | Corrosive/Lachrymatory |
| Hazard Class | 8 |
| Packing Group | III |
Introduction
2,5-Difluorobenzyl chloride is an organic compound with the chemical formula C7H5ClF2. It is a colorless and transparent liquid with a pungent odor.
2,5-Difluorobenzyl chloride has a wide range of applications in organic synthesis. It can be used as a reagent for the synthesis of other organic compounds, such as as an important intermediate synthesis of aromatic amines, aromatic ethers and aromatic alcohols and other organic compounds. In addition, it can also be used in the production of pesticides and drugs, and has important industrial and scientific research value.
Preparation of 2,5-Difluorobenzyl chloride can be obtained by reacting 2,5-difluorobenzyl alcohol with thionyl chloride. As the reaction proceeds, the alcohol reacts with thionyl chloride to give 2,5-difluorobenzyl sulfoxide, which then reacts with hydrogen chloride to give the target product, 2,5-Difluorobenzyl chloride.
Pay attention to safety when using 2,5-Difluorobenzyl chloride. It is irritating and corrosive and may cause damage to the eyes, skin and respiratory system. During operation, appropriate protective equipment, such as safety glasses, gloves and protective masks, should be worn to ensure a well-ventilated experimental environment. Avoid contact with oxidants, strong acids and strong bases. In case of contact or inhalation, please wash or seek medical help.


![dodecahydro-3,8,8,11a-tetramethyl-5H-3,5a-epoxynaphth[2,1-c]oxepin(CAS#57345-19-4)](https://www.xinchem.com/uploads/dodecahydro-38811a-tetramethyl-5H-35a-epoxynaphth.jpg)