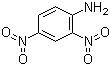
2,4-Dinitroaniline(CAS#97-02-9)
| Risk Codes | R26/27/28 – Very toxic by inhalation, in contact with skin and if swallowed. R33 – Danger of cumulative effects R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
| Safety Description | S28 – After contact with skin, wash immediately with plenty of soap-suds. S36/37 – Wear suitable protective clothing and gloves. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S28A - |
| UN IDs | UN 1596 6.1/PG 2 |
| WGK Germany | 2 |
| RTECS | BX9100000 |
| TSCA | Yes |
| HS Code | 29214210 |
| Hazard Class | 6.1 |
| Packing Group | II |
Introduction
2,4-Dinitroaniline is an organic compound. The following is an introduction to its properties, uses, manufacturing methods and safety information:
Quality:
- 2,4-Dinitroaniline is a yellow crystal that is insoluble in water.
- It has a high ignition point and explosiveness, and is classified as explosive.
- It can be reduced to amine compounds by strong bases and hydroxides.
Use:
- 2,4-Dinitroaniline is widely used in the chemical industry as a raw material for explosives and explosives.
- It can also be used in the synthesis of dyes and pigments, as well as as an important intermediate.
Method:
- The preparation of 2,4-dinitroaniline is usually carried out by nitrification. p-nitroaniline reacts with concentrated nitric acid to form 2,4-dinitronitroaniline, and then reduces the compound with strong acid to obtain 2,4-dinitroaniline.
Safety Information:
- 2,4-Dinitroaniline is a highly explosive chemical and should be kept away from open flames and heat sources.
- The risk of friction, impact, sparks, and electrostatic discharge needs to be strictly controlled during handling, storage, and transportation.
- Wear appropriate personal protective equipment such as safety glasses and protective gloves when in use. If ingested, seek medical attention immediately.
Always follow the relevant safety procedures when using and handling 2,4-dinitroaniline, and use it with knowledge and appropriate precautions.