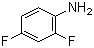
2,4-Difluoroaniline(CAS#367-25-9)
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S28 – After contact with skin, wash immediately with plenty of soap-suds. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. |
| UN IDs | UN 2941 6.1/PG 3 |
| WGK Germany | 1 |
| RTECS | BX3680000 |
| FLUKA BRAND F CODES | 8-9-23 |
| TSCA | T |
| HS Code | 29214210 |
| Hazard Note | Toxic |
| Hazard Class | 6.1 |
| Packing Group | III |
Introduction
2,4-Difluoroaniline is an organic compound. The following is an introduction to its properties, uses, manufacturing methods and safety information:Quality:2,4-Difluoroaniline is a colorless to pale yellow crystal with a peculiar odor. It is soluble in water and can also be soluble in most organic solvents.Uses: In chemical synthesis, it can be used as a derivative of amides, amide chain extenders and aromatic amines for the introduction of aniline groups in the synthesis process.Method:The preparation method of 2,4-difluoroaniline can be achieved by the reduction of p-fluorobenzene. A common method is to react fluorobenzene with sodium nitrite under acidic conditions to produce nitrosobenzene, which is then reduced to 2,4-difluoroaniline using a reducing agent such as iron powder.Safety Information:2,4-Difluoroaniline has certain toxicity under certain conditions. It is irritating and can cause damage to the eyes, skin, respiratory tract and digestive system. Appropriate personal protective equipment such as gloves and protective eyewear should be worn when using and handling this compound. It needs to be operated in a well-ventilated environment to avoid inhaling its vapors or coming into contact with its solutions.