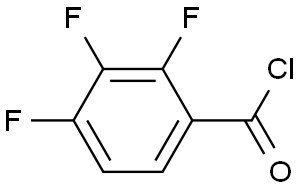
2,3,4-trifluorobenzoyl chloride(CAS#157373-08-5)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R36/37 – Irritating to eyes and respiratory system. |
| Safety Description | S23 – Do not breathe vapour. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S27 – Take off immediately all contaminated clothing. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| HS Code | 29163990 |
| Hazard Note | Corrosive/Lachrymatory |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
2,3,4-trifluorobenzoyl chloride, chemical formula C7H3ClF3O, is an organic compound. The following is a description of its nature, use, preparation and safety information:
Nature:
- 2,3,4-trifluorobenzoyl chloride is a colorless liquid with a pungent odor.
-Its melting point is about -52 ℃, and its boiling point is about 144 ℃.
-insoluble in water, but soluble in organic solvents such as ether, acetone and dichloromethane.
Use:
- 2,3,4-trifluorobenzoyl chloride can be used as an important reaction intermediate in organic synthesis.
-It is often used in the preparation of drugs and pesticides, especially for the manufacture of antibiotics, preservatives and pesticides.
-In organic synthesis, it can be used as an acylation reagent, esterification reagent and chlorination reagent.
Method:
-The preparation of 2,3,4-trifluorobenzoyl chloride is generally obtained by reacting benzoyl chloride with stannous trifluoride (SnF2) in the presence of cuprous chloride (CuCl).
-The reaction conditions are carried out at room temperature, and the resulting product is usually a mixture that requires further purification.
Safety Information:
- 2,3,4-trifluorobenzoyl chloride is irritating and should be avoided from skin, eyes and mucous membranes.
-Wear protective gloves, goggles and protective clothing during operation.
-Avoid inhaling its vapor during operation, and react in a well-ventilated place.
-In case of accidental contact or inhalation, rinse immediately with plenty of water and seek medical help.
Please note that the properties and preparation methods of chemical substances may be restricted by regions and regulations. Please read and comply with relevant safety technical guidelines and regulations carefully before use.