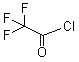
2,2,2-Trifluoroacetyl chloride(CAS#354-32-5)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R14 – Reacts violently with water R35 – Causes severe burns R37 – Irritating to the respiratory system |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3057 2.3 |
| WGK Germany | 2 |
| RTECS | AO7150000 |
| FLUKA BRAND F CODES | 4.5-19-21 |
| Hazard Note | Corrosive |
| Hazard Class | 2.3 |
Introduction
Trifluoroacetyl chloride is a colorless liquid with a strong pungent odor at room temperature. It is a highly reactive compound that reacts with moisture in water and air to form toxic gases. Trifluoroacetyl chloride is soluble in organic solvents such as acetone and ethanol. Trifluoroacetyl chloride is mainly used for acylation reactions in organic synthesis. It can be used as an acylation reagent to convert carboxylic acids into esters and generate acyl chloride. Trifluoroacetyl chloride can also be used as an acylation reagent, dehydrating agent, and reagent intermediate. One way to prepare trifluoroacetyl chloride is through the reaction of trifluoroacetic acid and thionyl chloride. This reaction is usually carried out at low temperatures and uses acids or oxidants as catalysts. Avoid direct contact with skin, eyes, and respiratory tract. During operation, appropriate protective measures such as wearing glasses, protective gloves and respiratory protective equipment need to be selected. It should work in a well-ventilated area to avoid its vapor accumulation. In the event of an accidental leak, it should be removed quickly and disposed of safely. It is important to use and store trifluoroacetyl chloride properly and to follow the handling and safe operating protocols of the relevant chemicals.