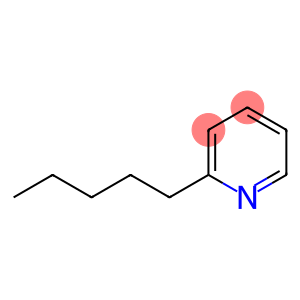
2-Pentyl Pyridine(CAS#2294-76-0)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29333990 |
| Hazard Note | Irritant |
Introduction
2-Amylpyridine is an organic compound. It is a colorless to pale yellow liquid with a special aromatic odor. Here are a few properties of 2-pentylpyridine:
Solubility: 2-pentylpyridine can be dissolved in water, alcohols and ether solvents, but insoluble in aliphatic hydrocarbons.
Stability: 2-Amylpyridine is relatively stable at room temperature, but may decompose or oxidize at high temperatures, pressures, or in contact with oxygen.
Flammability: 2-Penylpyridine has low flammability, but combustion can occur at high temperatures.
Uses of 2-Penylpyridine,:
Solvent: Due to its excellent solubility, 2-pentylpyridine is often used as a solvent in organic synthesis, especially in the synthesis of organometallic compounds.
Catalyst: 2-pentylpyridine can also be used as a catalyst for some organic reactions, such as carbonylation and amination.
There are two main methods for the preparation of 2-pentylpyridine:
Reaction of pyridine and pentanol: pyridine and pentanol are reacted under hydrogen catalysis to generate 2-pentylpyridine.
Reaction of pyridine and valeraldehyde: pyridine and valerdehyde are reacted under acidic conditions to form 2-pentylpyridine through condensation reaction.
Toxicity: 2-Penylpyridine is toxic, and direct contact with the skin, eyes, and respiratory tract should be prevented, and adequate ventilation should be ensured.
Combustion hazard: 2-Penylpyridine may cause fire at high temperatures, avoid contact with open flames and hot surfaces.
Storage and handling: 2-pentylpyridine should be stored in a cool, dry place, away from fire sources and oxidants, and handled and stored in accordance with relevant regulations.