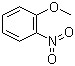
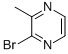
2-Nitroanisole(CAS#91-23-6)
| Hazard Symbols | T – Toxic |
| Risk Codes | R45 – May cause cancer R22 – Harmful if swallowed |
| Safety Description | S53 – Avoid exposure – obtain special instructions before use. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 2730 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | BZ8790000 |
| TSCA | Yes |
| HS Code | 29093090 |
| Hazard Class | 6.1 |
| Packing Group | III |
Introduction
2-nitroanisole, also known as 2-nitrophenoxymethane, is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of 2-nitroanisole:
Quality:
2-Nitroanisole is a colorless crystal or yellowish solid with a special smoky candle fragrance. At room temperature, it can be stable in the air. It is soluble in organic solvents such as ethanol, ether, and chloroform, but insoluble in water.
Use:
2-nitroanisole is mainly used as a chemical reagent in organic synthesis reactions. It can be used as a synthetic intermediate of aromatic compounds for the preparation of other compounds. It has a special scent of smoke candles and is also used as an ingredient in spices.
Method:
The preparation of 2-nitroanisole is generally obtained by the reaction of anisole with nitric acid. The specific preparation method is as follows:
1. Dissolve anisole in anhydrous ether.
2. Slowly add nitric acid dropwise to the solution, keep the reaction temperature between 0-5°C, and stir at the same time.
3. After the reaction, the inorganic salts in the solution are separated by filtration.
4. Wash and dry the organic phase with water and then purify it by distillation.
Safety Information:
2-Nitoanisole has an irritating effect on the eyes, skin, and respiratory tract and may cause itching, inflammation, and burns. Appropriate personal protective equipment such as chemical protective glasses, gloves, and protective clothing should be worn when used or prepared. It is explosive and should be avoided from contact with flammable substances, open flames and high-temperature environments. If the compound is inhaled or ingested, medical attention should be sought immediately.