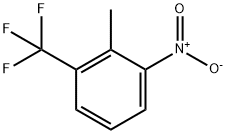
2-methyl-3-nitrobenzotrifluoride(CAS# 6656-49-1)
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. R25 – Toxic if swallowed R36/38 – Irritating to eyes and skin. R24/25 - |
| Safety Description | S23 – Do not breathe vapour. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S60 – This material and its container must be disposed of as hazardous waste. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37 – Wear suitable protective clothing and gloves. S20 – When using, do not eat or drink. S36 – Wear suitable protective clothing. S28 – After contact with skin, wash immediately with plenty of soap-suds. |
| UN IDs | 2810 |
| HS Code | 29049090 |
| Hazard Note | Harmful |
| Hazard Class | 6.1 |
| Packing Group | III |
Introduction
2-methyl-3-nitrotrifluorotoluene is an organic compound. The following is an introduction to its nature, use, manufacturing methods and safety information:
Quality:
- Appearance: White crystalline solid
- Solubility: Soluble in organic solvents such as ether, methanol and dimethyl sulfoxide
Use:
- It can also be used as a reagent in organic synthesis reactions, such as a source of nitrous acid and sulfur dioxide.
Method:
- MTF is usually prepared by nitrification and fluorine substitution of benzoic acid. First, benzoic acid is nitrified to obtain 2-nitrobenzoic acid, and then the carboxyl group in nitrobenzoic acid is substituted into trifluoromethyl group through fluorine gas substitution reaction.
Safety Information:
- MTF has certain toxicity and may cause harm to the human body, so it is necessary to pay attention to safety when using and operating.
- Contact with skin, inhalation, or accidental ingestion may cause irritation and injury, and appropriate precautions should be taken if necessary.
- Avoid contact with strong oxidizing agents and combustibles to prevent fire or explosion.
- When using and storing, follow the relevant safety operating procedures.