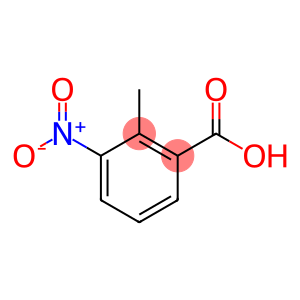
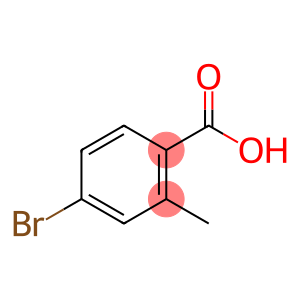
2-Methyl-3-nitrobenzoic acid(CAS#1975-50-4)
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S22 – Do not breathe dust. |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29163900 |
| Hazard Note | Irritant |
Introduction
2-Methyl-3-nitrobenzoic acid is an organic compound. The following is an introduction to its nature, use, preparation method and safety information:Quality:- Appearance: 2-Methyl-3-nitrobenzoic acid is a pure white crystal or light yellow powder.- Solubility: It has a low solubility in water but can be dissolved in organic solvents such as ethanol and chloroform.- Chemical properties: It is an acidic compound that reacts with alkali to form the corresponding salt.Use:- Chemical reagents: 2-methyl-3-nitrobenzoic acid is often used as an intermediate and chemical reagent in organic synthesis.Method:2-Methyl-3-nitrobenzoic acid can be prepared by the reaction of benzoic acid and nitrous acid. Under acidic conditions, nitrous acid is formed into nitrous ions (NO2-). Then, 2-methyl-3-nitrobenzoic acid is formed by the reaction of nitrous ions with benzoic acid.Safety Information:- 2-Methyl-3-nitrobenzoic acid is an organic compound and should be strictly followed by laboratory safety operating procedures.- Avoid direct contact with skin and eyes during use, storage, and handling.- Wear protective gloves, protective glasses, and a lab coat when handling and using 2-methyl-3-nitrobenzoic acid.- When used in a laboratory or industrial setting, ensure that there is a good ventilation system in place to prevent the accumulation of gases or vapors.- In case of accidental inhalation, ingestion, or skin contact, seek immediate medical attention and provide product packaging or labeling to a physician.