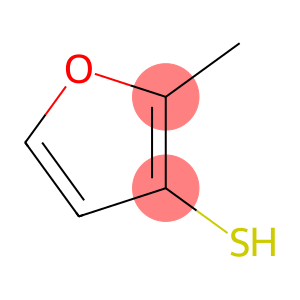
2-Methyl-3-furanthiol(CAS#28588-74-1)
| Risk Codes | R10 – Flammable R25 – Toxic if swallowed R36 – Irritating to the eyes R26 – Very Toxic by inhalation R2017/10/25 - |
| Safety Description | S16 – Keep away from sources of ignition. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S39 – Wear eye / face protection. S38 – In case of insufficient ventilation, wear suitable respiratory equipment. S28 – After contact with skin, wash immediately with plenty of soap-suds. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | UN 1228 3/PG 3 |
| WGK Germany | 3 |
| RTECS | LU6235000 |
| HS Code | 29321900 |
| Hazard Class | 3.2 |
| Packing Group | III |
Introduction
2-Methyl-3-mercaptofuran.
Quality:
- Appearance: Colorless liquid
- Solubility: Soluble in water and organic solvents such as alcohols and ethers.
Use:
- 2-Methyl-3-mercaptofuran is commonly used as an intermediate in organic synthesis.
- In organic synthesis, it is often used as a source of sulfides.
- 2-Methyl-3-mercaptofuran can also be used as a complexing agent and reducing agent for metal ions.
Method:
The common preparation method of 2-methyl-3-mercaptofuran is to react 2-methylfuran with sulfur ions at high temperatures.
Safety Information:
- 2-Methyl-3-mercaptofuran is irritating to the eyes and skin and should be rinsed with plenty of water immediately after contact.
- Appropriate personal protective equipment such as chemical goggles, gloves and gowns are required during operation.
- Avoid contact with oxidizing agents during storage and use to prevent dangerous situations such as fire or explosion.
- When using it for organic synthesis reactions, it needs to be carried out in a well-ventilated laboratory environment to reduce potential harm to the human body and environmental pollution.