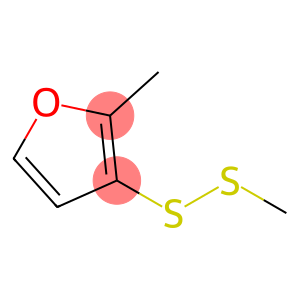
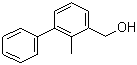
2-Methyl-3-biphenylmethanol(CAS#76350-90-8)
2-Methyl-3-biphenylmethanol(CAS#76350-90-8)
quality
Benzyl alcohol, also known as phenol. Here are some of the properties of biphenyl alcohol:
1. Physical properties: Biphenyl alcohol is a colorless crystal with a special phenolic taste. It is soluble in water, ethanol and ether solvents, and is liquid at room temperature.
2. Chemical properties: Phenyl alcohol is an acidic substance that can react with alkali to form corresponding salts. Under the action of a strong oxidizing agent, biphenyl alcohol can be oxidized to benzoquinone. It can also form quinones through phenolic condensation reactions.
3. Oxidation: Benzyl alcohol can be oxidized into benzoquinone by oxidants such as nitric oxide.
4. Flammability: Phenyl alcohol releases toxic fumes and combustion products when burned.
5. Chemical reaction: Diphenyl alcohol can undergo substitution reaction, and its hydroxyl group can be substituted to form different substituted phenols. Diphenyl alcohol can also be esterified to form ester compounds.
Uses and synthesis methods
Biphenyl alcohol is an organic compound. It is commonly used as a solvent and intermediate and has a wide range of uses in many industrial and laboratory applications.
The main uses of biphenyl alcohol include:
1. Solvent: Phenyl alcohol can dissolve many organic compounds and is often used as a reaction solvent or extraction solvent.
2. Synthetic chemistry: Biphenyl alcohol is a key intermediate in the synthesis of many important organic compounds. It can be used to synthesize ethers, esters, carboxylic acids, acid chloride and other compounds.
3. Nickel battery: Biphenyl alcohol can be used as a cathode material for nickel batteries.
There are many methods for the synthesis of biphenyl alcohol, among which the following are commonly used:
1. Benzyl alcohol oxidation method: benzyl alcohol and oxygen are oxidized in the presence of a catalyst to generate biphenyl alcohol.
2. Condensation reaction of bromobenzene and methanol: bromobenzene and methanol are reacted under alkaline conditions to produce biphenyl alcohol.
3. Benzoaldehyde reduction method: benzaldehyde reacts with a reducing agent such as hydrogen in the presence of a catalyst to generate biphenyl alcohol.


![[(difluoromethyl)thio]benzene (CAS# 1535-67-7)](https://www.xinchem.com/uploads/difluoromethylthiobenzene.png)