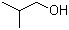
2-Methyl-1-propanol(CAS#78-83-1)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R10 – Flammable R37/38 – Irritating to respiratory system and skin. R41 – Risk of serious damage to eyes R67 – Vapors may cause drowsiness and dizziness |
| Safety Description | S13 – Keep away from food, drink and animal foodstuffs. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S37/39 – Wear suitable gloves and eye/face protection S46 – If swallowed, seek medical advice immediately and show this container or label. S7/9 - |
| UN IDs | UN 1212 3/PG 3 |
| WGK Germany | 1 |
| RTECS | NP9625000 |
| TSCA | Yes |
| HS Code | 29051990 |
| Hazard Class | 3 |
| Packing Group | III |
| Toxicity | LD50 orally in rats: 2.46 g/kg (Smyth) |
Introduction
Isobutanol, also known as 2-butanol. The following is an introduction to the properties, uses, preparation methods and safety information of isobutanol: 1. Properties: It is soluble in water and most organic solvents, and has strong dehydration and corrosiveness. 2. Uses: Isobutanol has a wide range of applications in industry. It is an important solvent for organic synthesis, which can be used for organic synthesis reactions and the preparation of a variety of chemical reagents. 3. Preparation method: Isobutanol can be obtained through a series of chemical reactions. Among them, the most commonly used method is prepared by the hydrogenation reaction of isobutylene. Catalyzed by a hydrogenation agent, such as nickel, isobutylene is reacted with hydrogen to produce isobutanol. 4. Safety information: Isobutanol is harmful to both people and the environment. It is a flammable liquid that explodes when in contact with an open flame, high temperatures, and oxygen. Isobutanol is irritating and corrosive, causing irritation and burns in contact with the skin, eyes, and respiratory tract. When using or storing isobutanol, care must be taken to avoid contact with fire and oxygen, and to pay attention to personal protective measures such as wearing protective gloves, goggles, and face shields. When disposing of waste, it is necessary to comply with relevant laws and regulations to avoid polluting the environment.