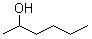
2-Hexanol(CAS#626-93-7)
| Risk Codes | R10 – Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. R22 – Harmful if swallowed |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. |
| UN IDs | UN 2282 3/PG 3 |
| WGK Germany | 1 |
| RTECS | MO8470000 |
| TSCA | Yes |
| HS Code | 29051990 |
| Hazard Class | 3.2 |
| Packing Group | III |
Introduction
2-Hexanol is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of 2-hexanol:Quality:3. Density: 0.810 g/cm³4. Soluble in water and some organic solvents such as ethanol and ether.Use:1. 2-Hexanol can be used as a solvent and extractant, which is widely used in the chemical industry.2. It can be used as a raw material for synthetic fragrances and flavors to give products a unique taste.3. 2-Hexanol can also be used in the preparation of plastics, resins, dyes, softeners, etc.Method:2-Hexanol is usually obtained by hydrohydrolysis of caproate. Caproate undergoes a catalytic hydrolysis reaction in the presence of hydrogen and a catalyst such as rhodium or nickel to produce 2-hexanol.Safety Information:1. 2-Hexanol is a low-toxicity compound, but care should still be taken to avoid contact with eyes, skin and inhalation.2. Wear appropriate personal protective equipment such as gloves, goggles, and protective clothing when using 2-hexanol.3. When storing, it should be sealed, away from fire sources and oxidants, and avoid reactions with strong acids, strong alkalis and oxidants.4. In case of accidental ingestion or inhalation, seek medical attention immediately.