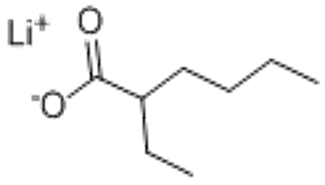
2-Ethyl-hexanoicacilithium salt (CAS# 15590-62-2)
Risk and Safety
Risk Codes R11 – Highly Flammable
R34 – Causes burns
R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
R65 – Harmful: May cause lung damage if swallowed
R67 – Vapors may cause drowsiness and dizziness
R38 – Irritating to the skin
Safety Description S9 – Keep container in a well-ventilated place.
S16 – Keep away from sources of ignition.
S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S29 – Do not empty into drains.
S33 – Take precautionary measures against static discharges.
S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection.
S60 – This material and its container must be disposed of as hazardous waste.
S61 – Avoid release to the environment. Refer to special instructions / safety data sheets.
S62 – If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label.
UN IDs UN 1206 3/PG 2
WGK Germany 1
TSCA Yes
Introduction
Lithium 2-ethylhexyl is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of lithium 2-ethylhexyl:
Quality:
- Appearance: Colorless or light yellow liquid
- Soluble: Soluble in non-polar solvents such as alkanes and aromatic hydrocarbons.
Use:
- Catalyst: 2-ethylhexyllithium can be used as a catalyst in some organic synthesis reactions, such as the exchange reaction of halogenated hydrocarbons and organolithium for the synthesis of various organic compounds.
- Heat stabilizer: It can be used as a heat stabilizer for plastics and rubber, which can improve their heat resistance.
- Conductive polymers: 2-ethylhexyl lithium can be used in the preparation of polymer electrolytes for use in electronic devices such as lithium-ion batteries and supercapacitors.
Method:
Lithium 2-ethylhexyl is generally synthesized by the following steps:
1. Magnesium hexyl bromide is reacted with ethyl acetate to obtain ethyl 2-hexylacetate.
2. Lithium acetate reacts with ethyl 2-hexyl acetate in the presence of tungsten chloride to generate 2-ethylhexyllithium.
Safety Information:
- Lithium 2-ethylhexyl should be kept away from high temperatures, ignition sources, and oxidizing agents, and avoid contact with moisture.
- Wear protective gloves and goggles to ensure good ventilation.
- Avoid inhaling its vapors or dust, and if you inhale too much, leave the contaminated area and breathe fresh air in time.
- Safe operating procedures should be followed during handling, storage and transportation.