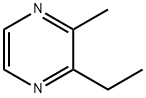
2-Ethyl-3-methyl pyrazine(CAS#15707-23-0)
| Risk Codes | R22 – Harmful if swallowed R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S24/25 – Avoid contact with skin and eyes. |
| UN IDs | UN 1993 3/PG 3 |
| WGK Germany | 3 |
| RTECS | UQ3335000 |
| TSCA | Yes |
| HS Code | 29339900 |
| Hazard Note | Irritant |
| Hazard Class | 3 |
| Packing Group | III |
| Toxicity | GRAS(FEMA)。 |
Introduction
2-Ethyl-3-methylpyrazine is an organic compound. The following is an introduction to its properties, uses, manufacturing methods and safety information:
Quality:
- Appearance: 2-ethyl-3-methylpyrazine is in a colorless liquid or solid crystalline form.
- Solubility: It has low solubility in water, but it can be soluble in organic solvents.
- Stability: It is a stable compound, but contact with strong oxidants and strong acids should be avoided.
Use:
- 2-Ethyl-3-methylpyrazine is a commonly used reagent and is also used as an intermediate in chemical synthesis.
Method:
2-Ethyl-3-methylpyrazine can be prepared by the following methods:
- Ethyl bromide is first reacted with pyrazine to generate 2-ethylpyrazine under alkaline conditions.
- Subsequently, 2-ethylpyrazine is reacted with methyl bromide to give the final 2-ethyl-3-methylpyrazine.
Safety Information:
- 2-Ethyl-3-methylpyrazine is generally considered to be of low toxicity, but proper safety protocols need to be followed.
- Avoid inhalation, contact with skin and eyes, and wear personal protective equipment such as gloves, goggles, and face shields.
- When storing and handling, keep it away from ignition sources and oxidizing agents to avoid the risk of fire and explosion.
- Refer to the relevant safety literature and the safety data sheets provided by the supplier for more detailed and accurate safety information.


![5-chlorobenzo[d]thiazol-2-amine(CAS#20358-00-3)](https://www.xinchem.com/uploads/5-chlorobenzo.gif)