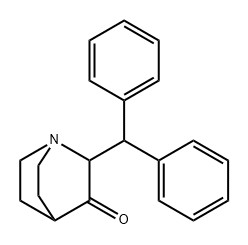
2-(DIPHENYLMETHYL)-QUINUCLIDIN-3-ONE(CAS#32531-66-1)
2-(DIPHENYLMETHYL)-QUINUCLIDIN-3-ONE, CAS NUMBER 32531-66-1, HAS MANY INTERESTING PROPERTIES IN CHEMISTRY AND RELATED APPLICATIONS.
From the analysis of the chemical structure, its unique molecular architecture fuses the structural parts of diphenyl methyl and quinine. Diphenyl methyl group brings a large steric hindrance and conjugation system, which affects the electron cloud flow of the molecule, while the quinine cyclic ketone part gives the molecule certain rigid and basic characteristics, and the two synergistically construct a relatively stable but reactive chemical structure. Typically in the form of a white crystalline powder, this solid form facilitates storage, transportation, and subsequent formulation processing. In terms of solubility, it has good solubility in non-polar organic solvents such as benzene and toluene, which is due to the non-polar region of the molecule, while it has poor solubility in more polar solvents such as water and alcohols, which is extremely critical for solvent selection, separation and purification steps in chemical synthesis.
In terms of medical application potential, its structure is similar to that of some existing psychotropic drugs, suggesting that it may act on central nervous system related targets. Early studies have shown that it may have a regulatory effect on the uptake and release of neurotransmitters, and is expected to be used in the treatment of psychiatric diseases such as schizophrenia and depression, and improve patients’ symptoms by intervening in abnormal nerve signaling. However, at present, most of them are in the stage of cell experiments and animal model exploration, and there is still a long way to go before they become clinical drugs, and it is necessary to deeply explore their pharmacological mechanisms, toxic side effects, pharmacokinetics and many other aspects.
From the perspective of synthesis process, it mainly relies on the fine organic synthesis route. Starting with relatively simple and easily available raw materials, the target molecule is constructed through complex reaction steps such as cyclization, substitution, and coupling. Researchers are constantly trying new catalysts and reaction media, optimizing reaction temperature, time and other conditions, and striving to improve synthesis efficiency and reduce costs, so as to ensure the feasibility of follow-up in-depth research and potential industrial production.