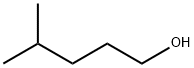
2-Bromopropionyl bromide(CAS#563-76-8)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R22 – Harmful if swallowed R34 – Causes burns |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29159000 |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
2-Bromopropionyl bromide is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of 2-bromopropionyl bromide:
Quality:
- Appearance: 2-Bromopropionyl bromide is a colorless to yellow liquid.
- Solubility: 2-Bromopropionyl bromide is insoluble in water, but soluble in some organic solvents such as ethanol and ether.
- Reactivity: 2-Bromopropionyl bromide has high electrophilicity and can undergo substitution reactions with nucleophiles.
Use:
- In laboratories and industries, 2-bromopropionyl bromide is often used as an organic synthesis reagent for the synthesis of other organic compounds.
- It can be used in the preparation of ketones, amides, and ester compounds.
Method:
- The preparation of 2-bromopropionyl bromide can be obtained by the reaction of 2-bromopropionic acid with silver bromide. The reaction is usually carried out in anhydrous conditions.
Safety Information:
- 2-Bromopropionyl bromide is a corrosive substance that can cause burns in contact with skin and eyes, and should be used with protective equipment.
- When using and storing, contact with oxidants and strong alkalis should be avoided to avoid dangerous reactions.