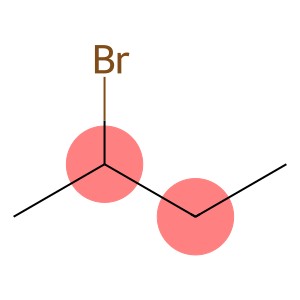
2-Bromobutane(CAS#78-76-2)
| Risk Codes | R11 – Highly Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. R10 – Flammable R52 – Harmful to aquatic organisms |
| Safety Description | S16 – Keep away from sources of ignition. S23 – Do not breathe vapour. S24/25 – Avoid contact with skin and eyes. S37/39 – Wear suitable gloves and eye/face protection S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | UN 2339 3/PG 2 |
| WGK Germany | 2 |
| RTECS | EJ6228000 |
| TSCA | Yes |
| HS Code | 29033036 |
| Hazard Note | Irritant/Highly Flammable |
| Hazard Class | 3 |
| Packing Group | II |
Introduction
2-Bromobutane is a halide alkane. The following is an introduction to some of its properties, uses, manufacturing methods, and safety information:
Quality:
- Appearance: Colorless liquid
- Solubility: Soluble in organic solvents, insoluble in water
Use:
- 2-Bromobutane, as a bromoalkanoid, is commonly used in organic synthesis reactions as an intermediate for carbon chain extension, introduction of halogen atoms, and preparation of other organic compounds.
- 2-Bromobutane can also be used as an additive in the coatings, glues and rubber industries.
Method:
- 2-Bromobutane can be prepared by reacting butane with bromine. The reaction can be carried out under light conditions or under heating.
Safety Information:
- 2-Bromobutane is irritating to the eyes, skin, and respiratory tract and can cause skin burns and eye damage.
- Inhaling too much can cause dizziness, difficulty breathing, and central nervous system depression.
- Wear appropriate protective equipment such as protective eyewear, gloves and respiratory protection when using 2-bromobutane.