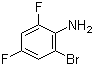
2-Bromo-4,6-difluoroaniline(CAS#444-14-4)
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R22 – Harmful if swallowed |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S25 – Avoid contact with eyes. |
| UN IDs | UN 1325 4.1/PG 2 |
| WGK Germany | 3 |
| HS Code | 29214200 |
| Hazard Note | Irritant |
| Hazard Class | 9 |
| Packing Group | III |
Introduction
2-Bromo-4,6-difluoroaniline is an organic compound. Its chemical formula is C6H3BrF2N. The following are some of the properties, uses, preparation and safety information of the compound:
Nature:
-Appearance: 2-Bromo-4,6-difluoroaniline is a colorless to pale yellow solid.
-Melting point: about 79-80 ℃.
-Boiling point: about 281 ℃.
-Solubility: It can be dissolved in some organic solvents, such as alcohols, ethers and organic ketones.
Use:
- 2-Bromo-4,6-difluoroaniline is commonly used as an intermediate in organic synthesis. It can be used to synthesize other compounds, such as fluorescent dyes and pesticides.
-The difluorophenyl and bromine groups of the compound can provide reactivity, making it suitable for participating in many substitution reactions.
Preparation Method:
- 2-Bromo-4,6-difluoroaniline can be synthesized by introducing bromine atoms into 4,6-difluoroaniline. A common method of synthesis is by introducing Br2 into the reaction with the addition of a suitable catalyst.
-The process of synthesizing this compound is dangerous, please follow the laboratory safety operation specifications.
Safety Information:
- 2-Bromo-4,6-difluoroaniline is an organic compound and should be treated with caution. It may cause irritation to the skin, eyes and respiratory tract.
-Wear appropriate personal protective equipment such as lab glasses, gloves and lab coats during operation.
-During handling and storage, avoid contact with oxidants or strong acids to avoid possible reactions or dangers.
-Comply with local and national safety standards and regulations during use or disposal. If used or handled incorrectly, it may cause harm to the human body and the environment.


![4-(Methoxycarbonyl)bicyclo[2.2.1]heptane-1-carboxylicacid (CAS# 15448-77-8)](https://www.xinchem.com/uploads/4Methoxycarbonylbicyclo221heptane1carboxylicacid.png)