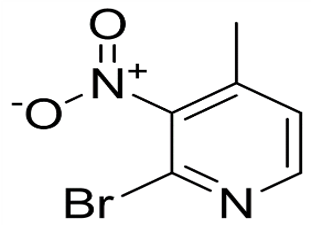
2-Bromo-4-methyl-3-nitropyridine(CAS# 23056-45-3)
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S37/39 – Wear suitable gloves and eye/face protection |
| Hazard Class | IRRITANT |
Introduction
It is an organic compound with the chemical formula of C, H, BrN, O. The following is an introduction to some of its properties, uses, methods and safety information:
Nature:
-Appearance: Colorless to yellow crystal or powder form.
-Solubility: It can be dissolved in organic solvents, such as ethanol, dimethyl sulfoxide and chloroform, but insoluble in water.
Use:
-Synthetic chemistry: It is a commonly used ligand, which can form complexes with transition metals and be used as catalysts in organic synthesis.
-Pesticide manufacturing: It can also be used as an intermediate for certain pesticides.
Method:
The preparation method can be carried out by the following steps:
1. First, lutidine is dissolved in dimethyl sulfoxide.
2. At low temperature, gradually add nitric acid while keeping the reaction temperature below 0 degrees Celsius.
3. Slowly add bromoethane Dropwise into the reaction system, continue to maintain low temperature, and stir until the end of the reaction.
4. Finally, the reaction mixture is filtered, washed, crystallized and dried to obtain calcium.
Safety Information:
It may cause certain hazards to the human body and the environment, so you need to pay attention to health and safety issues when using and handling. Here are some safety considerations:
-Wear appropriate protective equipment such as gloves, glasses and protective clothing when used.
-Avoid inhaling its dust and contact with skin. In case of accidental contact, immediately flush the affected area with plenty of water and seek medical help.
-Keep away from heat and fire sources during storage and handling, and maintain good ventilation.
-Avoid direct contact with oxidants, acids, alkalis and other substances to avoid dangerous reactions.
Please note that the information provided here is for reference only. When using and handling chemicals in practice, be sure to refer to relevant literature and handling safety regulations, and follow professional guidance.