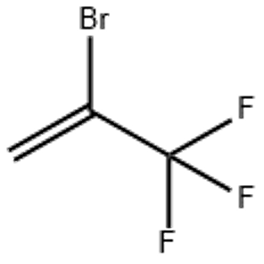
2-Bromo-3 3 3-trifluoropropene (CAS# 1514-82-5)
2-Bromo-3 3 3-trifluoropropene (CAS# 1514-82-5) introduction
2-bromo-3,3-trifluoropropene, also known as bromotrifluoroethylene. The following is an introduction to its properties, uses, manufacturing methods, and safety information:
nature:
2-bromo-3,3-trifluoropropene is a colorless and odorless gas. It has a higher density and is heavier than air.
Purpose:
2-bromo-3,3-trifluoropropene has a wide range of industrial applications. The main use of it is as a monomer for polymers, used for synthesizing high-performance materials such as polytetrafluoroethylene resin and polyfluoropropylene. It can also be used as a solvent, degradation agent, and extraction agent for special materials. In the electronics industry, 2-bromo-3,3-trifluoropropene is also widely used as a cleaning agent and insulation material in semiconductor manufacturing.
Manufacturing method:
2-bromo-3,3-trifluoropropene can be prepared by reacting trifluorochloroethylene with hydrogen bromide. During the reaction process, it is necessary to control the temperature and the ratio of reactants. For industrial production, it can be obtained by reacting fluorooxides with bromoalkanes.
Security information:
2-bromo-3,3-trifluoropropene is a hazardous material. It is a highly flammable gas that can form explosive mixtures with air and has a significant fire hazard to heat sources, sparks, open flames, etc. Fire and explosion prevention measures should be taken during handling and storage. When in contact with the skin and eyes, it may cause irritation and damage. When using, protective goggles and respirators should be worn, and good ventilation conditions should be ensured. If ingested or inhaled by mistake, seek medical assistance immediately. To ensure safety, users should carefully read and comply with relevant safety operating procedures.