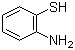
2-Aminothiophenol(CAS#137-07-5)
| Hazard Symbols | C – CorrosiveN – Dangerous for the environment |
| Risk Codes | R22 – Harmful if swallowed R34 – Causes burns R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
| Safety Description | S25 – Avoid contact with eyes. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 1760 |
2-Aminothiophenol(CAS#137-07-5)
Uses and synthesis methods
O-aminophenylthiophenol. Its common uses:
Dye field: o-aminophenol can be used as an intermediate of dyes for the synthesis of various organic dyes. Its structure contains amino and thiophenol groups, and different dye structural groups can be introduced through their functional group conversion reactions, so that different colors of dyes can be obtained.
Areas of treatment: Anthiophenol is an antibiotic that can be used to treat infectious diseases caused by certain bacteria. Its antibiotic action is based on interaction with the cell wall of bacteria and is able to interfere with the survival and replication processes of bacteria.
The synthesis method of o-aminophenthiophen can usually be carried out by the following steps:
Nitrophenylthiophenol is reacted with excess ammonia to form o-nitrothiophenol.
Reduction of o-nitrophenthionol to its corresponding o-aminothiophenol. Reducing agents are commonly used sodium sulfite, ammonium sulfite, etc.
In the laboratory, o-aminothiophenol can also be synthesized by other methods, such as the reaction of o-nitrophenol with amines to reduce nitrophenol. Different synthesis methods can be selected according to the needs and specific circumstances.